Abstract
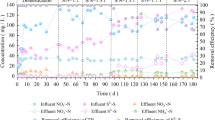
Nitrate addition stimulated sulfide oxidation by increasing the activity of nitrate-reducing sulfide-oxidizing bacteria (NR-SOB), decreasing the concentration of dissolved H2S in the water phase and, consequently, its release to the atmosphere of a pilot-scale anaerobic bioreactor. The effect of four different concentrations of nitrate (0.12, 0.24, 0.50, and 1.00 mM) was investigated for a period of 3 days in relation to sulfide concentration in two bioreactors set up at Guadalete wastewater treatment plant (Jerez de la Frontera, Spain). Physicochemical variables were measured in water and air, and the activity of bacteria implicated in the sulfur and nitrogen cycles was analyzed in the biofilms and in the water phase of the bioreactors. Biofilms were a net source of sulfide for the water and gas phases (7.22 ± 5.3 μmol s−1) in the absence of nitrate dosing. Addition of nitrate resulted in a quick (within 3 h) decrease of sulfide both in the water and atmospheric phases. Sulfide elimination efficiency in the water phase increased with nitrate concentrations following the Michaelis–Menten kinetics (K s = 0.63 mM NO3 −). The end of nitrate addition resulted in a recovery or increase of initial net sulfide production in about 3 h. Addition of nitrate increased the activity of NR-SOB and decreased the activity of sulfate-reducing bacteria. Results confirmed the role of NR-SOB on hydrogen sulfide consumption coupled with nitrate reduction and sulfate recycling, revealing Sulfurimonas denitrificans and Paracoccus denitrificans as NR-SOB of great importance in this process.







Similar content being viewed by others
References
Aelion, C. M., Rust, C. M., & Flora, J. R. V. (2000). Control of pH during denitrification in subsurface sediment microcosms using encapsulated phosphate buffer. Water Research, 34(5), 1447–1454.
Allen, L. A., Brooks, E., & Williams, I. L. (1949). Effect of treatment at the sewage works on the numbers and types of bacteria in sewage. Journal of Hygiene (Lond), 47(3), 303–319.
American Public Health Association, American Water Works Association, & Water Environment Federation. (2005). Standard methods for the examination of water and wastewater (21st ed.). Washington, DC: American Public Health Association.
Baker, S. C., Ferguson, S. J., Ludwig, B., Page, M. D., Richter, O. M., & van Spanning, R. J. (1998). Molecular genetics of the genus Paracoccus: Metabolically versatile bacteria with bioenergetic flexibility. Microbiology and Molecular Biology Reviews, 62(4), 1046–1078.
Beech, I. B., & Sunner, J. (2004). Biocorrosion: Towards understanding interactions between biofilms and metals. Current Opinion in Biotechnology, 15(3), 181–186. doi:10.1016/j.copbio.2004.05.001.
Boon, A. G. (1995). Septicity in sewers—Causes, consequences and containment. Water Science and Technology, 31(7), 237–253.
Cardoso, R. B., Sierra-Alvarez, R., Rowlette, P., Flores, E. R., Gomez, J., & Field, J. A. (2006). Sulfide oxidation under chemolithoautotrophic denitrifying conditions. Biotechnology and Bioengineering, 95(6), 1148–1157. doi:10.1002/bit.21084.
Cord-Ruwisch, R. (1985). A quick method for the determination of dissolved and precipitated sulfides in cultures of sulfate reducing bacteria. Journal of Microbiological Methods, 4(1), 33–36. doi:10.1016/0167-7012(85)90005-3.
Daly, K., Sharp, R. J., & McCarthy, A. J. (2000). Development of oligonucleotide probes and PCR primers for detecting phylogenetic subgroups of sulfate-reducing bacteria. Microbiology, 146(Pt 7), 1693–1705.
Eckford, R. E., & Fedorak, P. M. (2004). Using nitrate to control microbially-produced hydrogen sulfide in oil field waters. Petroleum Biotechnology: Developments and Perspectives, 151, 307–340.
Gadekar, S., Nemati, M., & Hill, G. A. (2006). Batch and continuous biooxidation of sulphide by Thiomicrospira sp. CVO: Reaction kinetics and stoichiometry. Water Research, 40(12), 2436–2446. doi:10.1016/j.watres.2006.04.007.
Garcia de Lomas, J., Corzo, A., Carmen Portillo, M., Gonzalez, J. M., Andrades, J. A., Saiz-Jimenez, C., et al. (2007). Nitrate stimulation of indigenous nitrate-reducing, sulfide-oxidising bacterial community in wastewater anaerobic biofilms. Water Research, 41(14), 3121–3131. doi:10.1016/j.watres.2007.04.004.
Garcia de Lomas, J., Corzo, A., Gonzalez, J. M., Andrades, J. A., Iglesias, E., & Montero, M. J. (2006). Nitrate promotes biological oxidation of sulfide in wastewaters: Experiment at plant-scale. Biotechnology and Bioengineering, 93(4), 801–811. doi:10.1002/bit.20768.
Gevertz, D., Telang, A. J., Voordouw, G., & Jenneman, G. E. (2000). Isolation and characterization of strains CVO and FWKO B, two novel nitrate-reducing, sulfide-oxidizing bacteria isolated from oil field brine. Applied and Environmental Microbiology, 66(6), 2491–2501.
Grasshoff, K., Ehrhardt, M., Kremling, K., & Almgren, T. (1983). Methods of seawater analysis. Weinheim: Verlag Chemie (2nd. revised and extended edition).
Greene, E. A., Hubert, C., Nemati, M., Jenneman, G. E., & Voordouw, G. (2003). Nitrite reductase activity of sulphate-reducing bacteria prevents their inhibition by nitrate-reducing, sulphide-oxidizing bacteria. Environmental Microbiology, 5(7), 607–617.
Gutierrez, O., Sutherland-Stacey, L., & Yuan, Z. (2010). Simultaneous online measurement of sulfide and nitrate in sewers for nitrate dosage optimisation. Water Science and Technology, 61(3), 651–658. doi:10.2166/wst.2010.901.
Hubert, C., & Voordouw, G. (2007). Oil field souring control by nitrate-reducing Sulfurospirillum spp. that outcompete sulfate-reducing bacteria for organic electron donors. Applied and Environmental Microbiology, 73(8), 2644–2652. doi:10.1128/AEM.02332-06.
Hubert, C., Voordouw, G., & Mayer, B. (2009). Elucidating microbial processes in nitrate- and sulfate-reducing systems using sulfur and oxygen isotope ratios: The example of oil reservoir souring control. Geochimica Et Cosmochimica Acta, 73(13), 3864–3879. doi:10.1016/j.gca.2009.03.025.
Ito, T., Nielsen, J. L., Okabe, S., Watanabe, Y., & Nielsen, P. H. (2002). Phylogenetic identification and substrate uptake patterns of sulfate-reducing bacteria inhabiting an oxic–anoxic sewer biofilm determined by combining microautoradiography and fluorescent in situ hybridization. Applied and Environmental Microbiology, 68(1), 356–364.
Jenneman, G. E., McInerney, M. J., & Knapp, R. M. (1986). Effect of nitrate on biogenic sulfide production. Applied and Environmental Microbiology, 51(6), 1205–1211.
Jiang, G., Sharma, K. R., Guisasola, A., Keller, J., & Yuan, Z. (2009). Sulfur transformation in rising main sewers receiving nitrate dosage. Water Research, 43(17), 4430–4440. doi:10.1016/j.watres.2009.07.001.
Leitao, R. C., van Haandel, A. C., Zeeman, G., & Lettinga, G. (2006). The effects of operational and environmental variations on anaerobic wastewater treatment systems: A review. Bioresource Technology, 97(9), 1105–1118. doi:10.1016/j.biortech.2004.12.007.
Linak, W. P., & Kramlich, J. C. (1998). A review of nitrous oxide behavior in the atmosphere, and in combustion and industrial systems. In T. Schneider (Ed.), Studies in environmental science (vol. 72, pp. 265–313). Amsterdam: Elsevier.
Mohanakrishnan, J., Gutierrez, O., Sharma, K. R., Guisasola, A., Werner, U., Meyer, R. L., et al. (2009). Impact of nitrate addition on biofilm properties and activities in rising main sewers. Water Research, 43(17), 4225–4237. doi:10.1016/j.watres.2009.06.021.
Mohanakrishnan, J., Kofoed, M. V., Barr, J., Yuan, Z., Schramm, A., & Meyer, R. L. (2011). Dynamic microbial response of sulfidogenic wastewater biofilm to nitrate. Applied Microbiology and Biotechnology, 91(6), 1647–1657. doi:10.1007/s00253-011-3330-3.
Molin, S., & Givskov, M. (1999). Application of molecular tools for in situ monitoring of bacterial growth activity. Environmental Microbiology, 1(5), 383–391.
Moraes, B. S., Souza, T. S., & Foresti, E. (2011). Characterization and kinetics of sulfide-oxidizing autotrophic denitrification in batch reactors containing suspended and immobilized cells. Water Science and Technology, 64(3), 731–738.
Neefs, J. M., Van de Peer, Y., Hendriks, L., De Wachter, R. (1990). Compilation of small ribosomal subunit RNA sequences. Nucleic Acids Research, 18 Suppl, 2237–2317.
Nemati, M., Jenneman, G. E., & Voordouw, G. (2001a). Impact of nitrate-mediated microbial control of souring in oil reservoirs on the extent of corrosion. Biotechnology Progress, 17(5), 852–859. doi:10.1021/bp010084v.
Nemati, M., Mazutinec, T. J., Jenneman, G. E., & Voordouw, G. (2001b). Control of biogenic H(2)S production with nitrite and molybdate. Journal of Industrial Microbiology and Biotechnology, 26(6), 350–355.
Nielsen, P. H. (1987). Biofilm dynamics and kinetics during high-rate sulfate reduction under anaerobic conditions. Applied and Environmental Microbiology, 53(1), 27–32.
Oh, S. E., Kim, K. S., Choi, H. C., Cho, J., & Kim, I. S. (2000). Kinetics and physiological characteristics of autotrophic denitrification by denitrifying sulfur bacteria. Water Science and Technology, 42(3–4), 59–68.
Okabe, S., Santegoeds, C. M., & De Beer, D. (2003). Effect of nitrite and nitrate on in situ sulfide production in an activated sludge immobilized agar gel film as determined by use of microelectrodes. Biotechnology and Bioengineering, 81(5), 570–577. doi:10.1002/bit.10495.
Poduska, R. A., & Anderson, B. D. (1981). Successful storage lagoon odor control. Journal Water Pollution Control Federation, 53(3), 299–310.
Roberts, D. J., Nica, D., Davis, J. L., Kirby, L., & Zuo, G. (2000). Isolation and characterization of microorganisms involved in the biodeterioration of concrete in sewers. International Biodeterioration & Biodegradation, 46(1), 61–68.
Rutledge, R. G. (2004). Sigmoidal curve-fitting redefines quantitative real-time PCR with the prospective of developing automated high-throughput applications. Nucleic Acids Research, 32(22), e178. doi:10.1093/nar/gnh177.
Snaidr, J., Amann, R., Huber, I., Ludwig, W., Schleifer, K. H. (1997). Phylogenetic analysis and in situ identification of bacteria in activated sludge. Appl Environ Microbiol, 63(7), 2884–2896.
Tang, K., Baskaran, V., & Nemati, M. (2009). Bacteria of the sulphur cycle: An overview of microbiology, biokinetics and their role in petroleum and mining industries. Biochemical Engineering Journal, 44(1), 73–94. doi:10.1016/j.bej.2008.12.011.
Vaiopoulou, E., Melidis, P., & Aivasidis, A. (2005). Sulfide removal in wastewater from petrochemical industries by autotrophic denitrification. Water Research, 39(17), 4101–4109. doi:10.1016/j.watres.2005.07.022.
Van Rijn, J., Tal, Y., & Schreier, H. J. (2006). Denitrification in recirculating systems: Theory and applications. Aquacultural Engineering, 34(3), 364–376. doi:10.1016/j.aquaeng.2005.04.004.
Voordouw, J. K., & Voordouw, G. (1998). Deletion of the rbo gene increases the oxygen sensitivity of the sulfate-reducing bacterium Desulfovibrio vulgaris Hildenborough. Applied and Environmental Microbiology, 64(8), 2882–2887.
Yuan, Z. G., Jiang, G. M., Gutierrez, O., & Sharma, K. R. (2010). Effects of nitrite concentration and exposure time on sulfide and methane production in sewer systems. Water Research, 44(14), 4241–4251. doi:10.1016/j.watres.2010.05.030.
Zhang, J. Z., & Fischer, C. J. (2006). A simplified resorcinol method for direct spectrophotometric determination of nitrate in seawater. Marine Chemistry, 99(1–4), 220–226. doi:10.1016/j.marchem.2005.09.008.
Acknowledgments
We acknowledge the support of the grants P06-RNM-01787, P11-RNM-7199, the PAI groups RNM-214 and BIO-288 from Consejería de Innovación, Ciencia y Empresa, Junta de Andalucía, Spain and CTM2009-10736 from the Ministerio de Innovación y Ciencia, Spain, which include cofinancing from FEDER funds. S. Papaspyrou was funded by a JAE-Doc fellowship (Programa JAE, JAE-Doc109, Spanish National Research Council) and a Marie Curie ERG action (NITRICOS, 235005, European Union). We also want to acknowledge the personnel from Guadalete WWTP and AJEMSA for the technical help and providing us the physicochemical analysis of raw wastewater during 2008 and 2009 and E. Iglesias from Yara Iberian for providing nitrate.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Villahermosa, D., Corzo, A., González, J.M. et al. Reduction of Net Sulfide Production Rate by Nitrate in Wastewater Bioreactors. Kinetics and Changes in the Microbial Community. Water Air Soil Pollut 224, 1738 (2013). https://doi.org/10.1007/s11270-013-1738-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-013-1738-3