Abstract
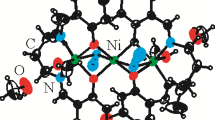
Nickel(II) complexes with 2,3-dihydroxybenzaldehyde N4-substituted thiosemicarbazone ligands (H3L1–H3L4) have been synthesized and characterized with the aim of evaluating the effect of N4 substitution in the thiosemicarbazone moiety on their coordination behavior and biological activities. Two series of nickel(II) complexes with the general formulae [Ni(H3L)(H2L)]ClO4 and [Ni2(HL)2] were characterized by analytical and spectral techniques. The molecular structure of one of the complexes, namely, [Ni(H3L4)(H2L4)]ClO4 was established by single crystal X-ray diffraction studies. The crystal structure of this complex revealed that two H3L4 ligands are coordinated to nickel(II) in different modes; one as a neutral tridentate ONS ligand and the other is as a monoanionic tridentate (ONS−) ligand. The antimicrobial activities of the compounds were tested against 25 bacterial strains via the disc diffusion method, and their minimum inhibitory concentration (MIC) and minimum microbicidal concentration were evaluated using microdilution methods. With a few exceptions, most of the compounds exhibited low-to-moderate inhibitory activities against the tested bacterial strains. However, the complexes [Ni2(HL3)2] (7) and [Ni2(HL4)2] (8) indicated higher inhibitory activity against Salmonella enterica ATCC 9068 (MIC values 15.7 and <15.7 μg/ml, respectively), compared with gentamicin as the positive control (MIC 25 μg/ml). Complex (7) also inhibited Streptococcus pneumoniae more efficiently (MIC 31.2 μg/ml), compared with gentamicin (MIC > 50 μg/ml). The toxicities of the compounds were tested on brine shrimp (Artemia salina), where no meaningful toxicity level was noted for both the free ligands and the complexes. The cytotoxicities of the compounds on cell viability were determined on MCF7, PC3, A375, and H413 cancer cells in terms of IC50; complexes [Ni(H3L3)(H2L3)]ClO4 (3), [Ni2(HL3)2] (7) and [Ni2(HL4)2] (8) exhibited significant cytotoxicity on the tested cell lines.






Similar content being viewed by others
References
Hochreuther S, Puchta R, Eldik RV (2011) Inorg Chem 50:12747–12761
Tarafder MTH, Asmadi A, Talib SMS, Ali AM, Crouse KA (2001) Transition Met Chem 26:170–174
Baldini M, Belicchi-Ferrari M, Bisceglie F, Dall’Aglio PP, Pelosi G, Pinelli S, Tarasconi P (2004) Inorg Chem 43:7170–7179
Banerjee D, Yogeeswari P, Bhat P, Thomas A, Srividya M, Sriram D (2011) Eur J Med Chem 46:106–121
Campbell MJM, Morrison E, Rogers V (1987) Polyhedron 6:1703–1705
Casas JS, Garcìa-Tasende MS, Sordo J (2000) J Coord Chem Rev 209:197–261
Ramachandran E, Raja DS, Mike JL, Wagner TR, Zeller M, Natarajan K (2012) RSC Adv 2:8515–8525
Afrasiabi Z, Sinn E, Padhye S, Dutta S, Padhye S, Newton C, Anson CE, Powell AK (2003) J Inorg Biochem 95:306–314
Liberta AE, West DX (1992) Biometals 5:121–126
Scovill JP, Klayman DL, Franchino FC (1982) J Med Chem 25:12611264
Dilovic I, Rubcic M, Vrdoljak V, Kraljevic SP, Kralj M, Piantanida I, Cindric M (2008) Bioorg Med Chem 16:5189–5198
Kovala-Demertzi D, Domopoulou A, Demertzis MA, Papageorgiou A, West DX (1997) Polyhedron 16:3625–3633
Marzano C, Pellei M, Tisato F, Santini C (2009) Anticancer Agents Med Chem 9:185–211
Pelosi G (2010) Open Crystallogr J 3:16–28
Bindu P, Kurup MRP, Satyakeerty TR (1998) Polyhedron 18:321–331
Prabhakaran R, Kalaivani P, Huang R, Poornima P, Padma VV, Dallemer FKN (2013) J Biol Inorg Chem 18:233–247
Basuli F, Peng SM, Bhattacharya S (2000) Inorg Chem 39:1120–1127
Andrews RK, Blakeley RL, Zerner B (1988) In: Sigel H, Sigel A (eds) Metal ions in biological systems, vol 23. Marcel Dekker, New York
Skyrianou KC, Perdih F, Turel I, Kessissoglou DP, Psomas G (2010) J Inorg Biochem 104:740–749 (and references cited therein)
Swesi AT, Farina Y, Baba I (2007) Sains Malays 36:21–26
Tan KW, Seng HL, Lim FS, Cheah SC, Ng CH, Koo KS, Mustafa MR, Ng SW, Maah MJ (2012) Polyhedron 38:275–284
Zhu X, Wang C, Lu Z, Dang Y (1997) Transition Met Chem 22:9–13
Bruker (2009) APEX2 and SAINT. Bruker AXS, Madison
Sheldrick GM (2001) SHELXTL version 6.10. Bruker AXS Inc., Madison, Wisconsin, USA.
Farrugia LJ (1997) J Appl Cryst 30:565–566
Spek AL (2003) J Appl Cryst 36:7–13
Westrip SP (2010) J Appl Cryst 43:920–925
Andrews JM (2005) J Antimicrob Chemother 56:60–76
Bauer AW, Kirby WM, Sherris JC, Turck M (1966) Am J Clin Pathol 45:493–496
Espinel-Ingroff B, Iqbal AN, Ellis D, Pfaller MA, Messer S, Rinaldi M, Fothergill A, Gibbs DL, Wang A (2007) J Clin Microbiol 45:1811–1820
Sarker SD, Nahar L, Kumarasamy Y (2007) Methods 42:321–324
Banfi E, Scialino G, Monti-Bragadin C (2003) Antimicrob J Chemoter 52:796–800
Radic GP, Glodovic VV, Radojevic ID, Stefanovic OD, Comic LR, Rathovic ZR, Valkonen A, Rissanen K, Trifunovic SR (2012) Polyhedron 31:69–76
Rahman AU, Choudhary MI, Thomsen WJ (2001) Bioassay techniques for drug development. Harwood Academic, The Netherlands
Looi CY, Moharram B, Paydar M, Wong YL, Leong KH, Mohamad K, Arya A, Wong WF, Mustafa MR (2013) BMC Complement Altern Med 13:166
Mosmann T (1983) J Immunol Methods 65:55–63
West DX, Nassar AA, El-Saied FA, Ayad MI (1998) Transition Met Chem 23:423–427
Lobana TS, Kumari P, Hundal G, Butcher RJ (2010) Polyhedron 29:1130–1136
Afrasiabi Z, Sinn E, Lin W, Ma Y, Campana C, Padhye S (2005) J Inorg Biochem 99:1526–1531
Afrasiabi Z, Sinn E, Chen J, Ma Y, Rheingold AL, Zakharov LN, Rath N, Padhye S (2004) Inorg Chim Acta 357:271–278
Bindu P, Kurup MRP, Satyakeerty RT (1999) Polyhedron 18:321–331
Chandra S, Sharma AK (2009) Spectrochim Acta Part A 7:2851–2858
Affan MA, Salam MA, Ahmad FB, Ismail J, Shamsuddin MB, Ali HM (2011) Inorg Chim Acta 366:227–232
Barber DE, Lu Z, Richardson T, Crabtree RH (1992) Inorg Chem 31:4709–4711
Prabhakaran R, Sivasamy R, Angayarkanni J, Huang R, Kalaivani P, Karvembu R, Dallemer F, Natarajan K (2011) Inorg Chim Acta 374:647–653
Acknowledgments
The authors acknowledge the support from PPP (PS 484/2010B) and UMRG (RG206/11AFR) from University of Malaya (UM). The authors also would like to thank the Department of Chemistry, University of Malaya for facilities to carry out the research work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shawish, H.B., Paydar, M., Looi, C.Y. et al. Nickel(II) complexes of polyhydroxybenzaldehyde N4-thiosemicarbazones: synthesis, structural characterization and antimicrobial activities. Transition Met Chem 39, 81–94 (2014). https://doi.org/10.1007/s11243-013-9777-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11243-013-9777-6