Abstract
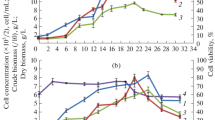
A cell suspension culture of Devil’s claw (Harpagophytum procumbens), a South African plant with high medicinal value, cultivated under submerged conditions showed stable growth and accumulated high amounts of biomass (18.2 g l−1). Flow cytometry analyses of the suspension’s cell cycle kinetics showed that proportions of cells in G0/G1 and S phases varied insignificantly (between 69–76% and 9–13%, respectively) during the cultivation, while the proportion of G2/M-phase cells increased until day 8 of cultivation, when the exponential phase of cell growth ended. Metabolite production in the culture was studied through simultaneous determination of three bioactive phenylethanoid glycosides (verbascoside, β-OH-verbascoside and leucosceptoside A) by high performance liquid chromatography. It was found that suspended Devil’s claw cells accumulated mainly verbascoside (517.3 mg l−1), followed by leucosceptoside A (107.1 mg l−1) and β-OH-verbascoside (80.3 mg l−1). In addition, several fatty acids and β-sitosterol were identified in the cell suspension by gas chromatographic-mass spectrometry analysis. Comparison of the results with previously acquired data for Harpagophytum procumbens transformed roots indicate that cell suspensions cultures are more promising as potential commercial sources of metabolites such as phenylethanoid glycosides.




Similar content being viewed by others
References
Dembitsky VM (2005) Astonishing diversity of natural surfactants: 5. Biologically active glycosides of aromatic metabolites. Lipids 40:869–900
Estrada-Zuniga ME, Cruz-Sosa F, Rodriguez-Monroy M, Verde-Calvo JR, Vernon-Carter EJ (2009) Phenylpropanoid production in callus and cell suspension cultures of Buddleja cordata Kunth. Plant Cell Tissues Organ Cult 97:39–47
Fu G, Pang H, Wong YH (2008) Naturally occurring phenylethanoid glycosides: potential leads for new therapeutics. Curr Med Chem 15:2592–2613
Georgiev M, Kuzeva S, Pavlov A, Kovacheva E, Ilieva M (2006) Enhanced rosmarinic acid production by Lavandula vera MM cell suspension culture through elicitation with vanadyl sulfate. Z Naturforsch 61c:241–244
Georgiev M, Weber J, Maciuk A (2009) Bioprocessing of plant cell cultures for mass production of targeted compounds. Appl Microbiol Biotechnol 83:809–823
Georgiev M, Alipieva K, Pashova S, Denev P, Angelova M, Kerns G, Bley Th (2010) Antioxidant activity of devil’s claw cell biomass and its active constituents. Food Chem 121:967–972
Gyurkovska V, Alipieva K, Maciuk A, Dimitrova P, Ivanovska N, Haas C, Bley Th, Georgiev M (2010) Anti-inflammatory activity of Devil’s claw in vitro systems and their active constituents. Food Chem (In press) DOI: 10.1016/j.foodchem.2010.08.056
Haas C, Weber J, Ludwig-Müller J, Deponte S, Bley Th, Georgiev M (2008) Flow cytometry and phytochemical analysis of a sunflower cell suspension culture in a 5-L bioreactor. Z Naturforsch 63c:699–705
Homova V, Weber J, Schulze J, Alipieva K, Bley Th, Georgiev M (2010) Devil’s claw hairy root culture in flasks and in a 3-L bioreactor: bioactive metabolite accumulation and flow cytometry. Z Naturforsch 65c:472–478
Jimenez C, Riguera R (1994) Phenylethanoid glycosides in plants: structure and biological activity. Nat Prod Rep 11:591–606
Linsmayer EM, Skoog F (1965) Organic growth factor requirements of tobacco tissue cultures. Physiol Plant 18:100–127
Loureiro J, Rodriguez E, Dolezel J, Santos C (2007) Two new nuclear isolation buffers for plant DNA flow cytometry: a test with 37 species. Ann Bot 100:875–888
Lu C-T, Mei X-G (2003) Improvement of phenylethanoid glycosides production by a fungal elicitor in cell suspension culture of Cistanche deserticola. Biotechnol Lett 25:1437–1439
Ludwig-Müller J, Georgiev M, Bley Th (2008) Metabolite and hormonal status of hairy root cultures of devil’s claw (Harpagophytum procumbens) in flasks and in a bubble column bioreactor. Process Biochem 43:15–23
Ovesna Z, Vachalkova A, Horvathova K (2004) Taraxasterol and β-sitosterol: new naturally compounds with chemoprotective/chemopreventive effects. Neoplasma 52:407–414
Pettit GR, Numata A, Takemura T, Ode RH, Narula AS, Schmidt JM, Cragg GM, Pase CP (1990) Antineoplastic agents, 107. Isolation of acteoside and isoacteoside from Castilleja linariaefolia. J Nat Prod 53:456–458
Roessner U, Willmitzer L, Fernie AR (2002) Metabolic profiling and biochemical phenotyping of plant systems. Plant Cell Rep 21:189–196
Suchismita D, Ramawat KG (2009) Elicitation of guggulsterone production in cell cultures of Commiphora wightii by plant gums. Plant Cell Tissue Organ Cult 96:349–353
Verpoorte R, Contin A, Memelink J (2002) Biotechnology for the production of plant secondary metabolites. Phytochem Rev 1:13–25
Yanpaisan W, King NJC, Doran PM (1998) Analysis of cell cycle activity and population dynamics in heterogeneous plant cell suspensions using flow cytometry. Biotechnol Bioeng 58:515–528
Yanpaisan W, King NJC, Doran PM (1999) Flow cytometry of plant cells with applications in large-scale bioprocessing. Biotechnol Adv 17:3–27
Zhou X, Zhong J-J (2010) Plant cell culture, secondary product accumulation. In: Flickinger MC (ed) Encyclopedia of industrial biotechnology: bioprocess. bioseparation and cell technology. Wiley, USA, pp 3883–3912
Acknowledgments
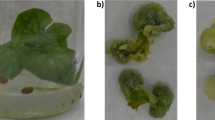
This research was supported by a grant from the National Science Fund of Bulgaria (contract number DO-02-261/2008). M.G. was supported by a short-term fellowship awarded by the Saxony Ministry of Science and Culture (SWMK). The authors express their thanks to Dr. G. Kerns (SIAB) for kindly supplying the Harpagophytum procumbens callus culture.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Stancheva, N., Weber, J., Schulze, J. et al. Phytochemical and flow cytometric analyses of Devil’s claw cell cultures. Plant Cell Tiss Organ Cult 105, 79–84 (2011). https://doi.org/10.1007/s11240-010-9844-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-010-9844-z