Abstract
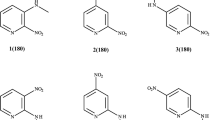
15N NMR chemical shifts of the exo- and endocyclic nitrogen atoms show how efficient is the ground-state intramolecular charge transfer between these sites in 4-dimethylamino-1-methylpyridinium cation (increased contribution of the quinoid resonance form results in a shielding and deshielding effect of their NMR signals, respectively). As it was anticipated, insertion of vinylene and/or 1,4-phenylene spacers to the cation considerably hinders the ground-state charge transfer. This hypothesis is further supported by an analysis of the C–NMe2 bond lengths (X-ray data show that spacers elongate this bond). The selected valence angles in the compounds studied are also linearly dependent on δ(15Nendo) and δ(15Nexo) values. Although the correlation coefficient for the δ(15Nendo) versus δ(15Nexo) dependence is equal to 0.983, decrease of the net charge on one nitrogen atom is not compensated entirely by its increase on another nitrogen atom. This shows that exocyclic nitrogen atom is not the only acceptor of the positive charge in the molecule. The natural population analysis shows that the positive charge is transferred not only to the exocyclic N but also to, e.g., 1- and N-methyl C as well as to C3 and C5 atoms in pyridine ring. Ground-state charge transfer through the p-phenylene moiety was found to be less effective than through the trans-vinylene bridge.



Similar content being viewed by others
References
Colapietro M, Dominicano A, Marciante C, Portalone G (1982) Z Naturforsch 37B:1309
Hiberty PC, Ohanessian G (1984) J Am Chem Soc 106:6963
Hammett LP (1970) Physical organic chemistry. McGraw-Hill, London
Krygowski TM, Maurin J (1989) J Chem Soc Perkin Trans 2:695
Hrobárik P, Horváth B, Sigmundová I, Zahradnik P, Malkina OL (2007) Magn Reson Chem 45:942
Marek R, Lyčka A, Kolehmainen E, Sievänen E, Toušek J (2007) Curr Org Chem 11:1154
Kalatzis E, Kiriazis L (1989) J Chem Soc Perkin Trans 2:179
Čopar A, Stanovnik B, Tišler M (1996) J Heterocycl Chem 33:465
Koenigs E, Ruppelt E (1934) Liebigs Ann Chem 509:142
Hutchings MG (1984) Tetrahedron 40:2061
Baumgarten HE (1953) J Am Chem Soc 75:1239
McEven WE, Terss RH, Elliott IW (1952) J Am Chem Soc 74:3605
Kost AN, Sheinkman AK, Kazarinova NF (1964) Zh Obshch Khim 34:2044
Gawinecki R, Bąk T, Rasała D, Styrcz S (1996) Polish J Chem 70:188
Sheinkman AK, Rudenko NZ, Kazarinova NF, Lysenko VB (1963) Zh Obshch Khim 33:1964
Gawinecki R, Trzebiatowska K (2000) Dyes Pigment 45:103
Kolehmainen E, Ośmiałowski B, Krygowski TM, Kauppinen R, Nissinen M, Gawinecki R (2000) J Chem Soc Perkin Trans 2:1259
Otwinowski Z, Minor W (1997) In: Carter CW, Sweet RM (eds) Methods in Enzymology, Vol. 276. Academic Press: New York, pp 307
Burla MC, Camalli M, Carrozzini B, Cascarano GL, Giacovazzo C, Polidori G, Spagna J (2003) J Appl Cryst 36:1103
Sheldrick GM (1997) SHELXL-97. University of Göttingen, Germany
Reed AE, Curtiss LA, Weinhold F (1988) Chem Rev 88:899
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Montgomery Jr. JA, Vreven T, Kudin KN, Burant JC, Millam JM, Iyengar SS, Tomasi J, Barone V, Mennucci B, Cossi M, Scalmani G, Rega N, Petersson GA, Nakatsuji H, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Klene M, Li X, Knox JE, Hratchian HP, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Ayala PY, Morokuma K, Voth GA, Salvador P, Dannenberg JJ, Zakrzewski VG, Dapprich S, Daniels AD, Strain MC, Farkas O, Malick DK, Rabuck AD, Raghavachari K, Foresman JB, Ortiz JV, Cui Q, Baboul AG, Clifford S, Cioslowski J, Stefanov BB, Liu G, Liashenko A, Piskorz P, Komaromi I, Martin RL, Fox DJ, Keith T, Al-Laham MA, Peng CY, Nanayakkara A, Challacombe M, Gill PMW, Johnson B, Chen W, Wong MW, Gonzalez C, Pople JA (2004) Gaussian 03 Revision D.02, Gaussian, Inc., Wallingford
Rodig OR (1974) In: Abramovitch RA (ed) Pyridine and its derivatives, supplement part one. Interscience Publication, New York, pp 309
Pearson RG, Songstad J (1967) J Am Chem Soc 89:1827
Le Noble WJ (1974) Highlights of organic chemistry. An advanced textbook. Marcel Dekker, New York, p 848
Coe BJ, Harris JA, Asselberghs I, Wostyn KW, Clays K, Persoons A, Brunschwigh BS, Coles SJ, Th Gelbrich, Light ME, Hursthouse MB, Nakatani K (2003) Adv Funct Mater 13:347
Cao X, Tolbert RW, McHale JL, Edwards WD (1998) J Phys Chem A 102:2739
Gawinecki R, Trzebiatowska K (2001) Polish J Chem 75:231
Sibi MP, Lichter RL (1977) J Org Chem 42:2999
Dorie J, Mechin B, Martin G (1979) Org Magn Reson 12:229
Mishra A, Behera RK, Fronczek FR, Vidyasagar M, Behera GB (1999) Indian J Chem 38B:982
Ren P, Qin J-G, Zhang D-Q, Hu H-M (2004) Chin J Struct Chem 23:735
OYa Borbulevych, Clark RD, Romero A, Tan L, MY Antipin, Nesterov VN, Carselino BH, Moore CE, Sanghadasa M, Timofeeva TV (2002) J Mol Struct 604:73
Tishkov AT, Dilman AD, Faustov VI, Birukov AA, Lysenko KS, Belyakov PA, Ioffe SL, Strelenko YA, Myu A (2002) J Am Chem Soc 124:11358
Graham EM, Miskowski VM, Perry JW, Coulter DR, Stiegman AE, Schaefer WP, Marsh RE (1989) J Am Chem Soc 111:8771
Wouters J, Evrard G, Durant F, Kalgutkar A, Castagnoli N (1996) Acta Cryst C52:1033
Glavcheva Z, Umezawa H, Okada Sh, Nakanishi H (2004) Mater Lett 58:2466
Acknowledgments
We are very much indebted to the ACK CYFRONET AGH, Kraków (MNiSW/SGI3700/UTPBydg/042/2007) and CI TASK Gdańsk, for supply of computer time and providing programs.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Gawinecki, R., Stanovnik, B., Valkonen, A. et al. Effect of vinylene and 1,4-phenylene spacers on efficiency of the ground-state intramolecular charge-transfer in enlarged 4-dimethylamino-1-methylpyridinium cations. Struct Chem 20, 655–662 (2009). https://doi.org/10.1007/s11224-009-9457-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-009-9457-5