ABSTRACT
Purpose
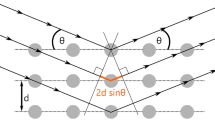
To investigate solid state transformations of drug substances during compaction using grazing incidence X-ray diffraction (GIXD).
Methods
The solid forms of three model drugs–theophylline (TP), nitrofurantoin (NF) and amlodipine besylate (AMB)–were compacted at different pressures (from 100 to 1000 MPa); prepared tablets were measured using GIXD. After the initial measurements of freshly compacted tablets, tablets were subjected to suitable recrystallization treatment, and analogous measurements were performed.
Results
Solid forms of TP, NF and AMB showed partial amorphization as well as crystal disordering during compaction; the extent of these effects generally increased as a function of pressure. The changes were most pronounced at the outer surface region. The different solid forms showed difference in the formation of amorphicity/crystal disordering. Dehydration due to compaction was observed for the TP monohydrate, whereas hydrates of NF and AMB were stable towards dehydration.
Conclusions
With GIXD measurements, it was possible to probe the solid form composition at the different depths of the tablet surfaces and to obtain depth-dependent information on the compaction-induced amorphization, crystal disordering and dehydration.






Similar content being viewed by others
REFERENCES
Alderborn G, Nyström C. Pharmaceutical powder compaction technology. New York: Marcel Dekker, Inc.; 1995.
Hilfiker R, Blatter F, von Raumer M. Relevance of solid-state properties for pharmaceutical products. In: Hilfiker R, editor. Polymorphism: in the pharmaceutical industry. Weinheim: WILLEY-VCH Verlag GmbH & Co.; 2006. p. 1–20.
Joiris E, Martino PD, Berneron C, Guyot-Hermann A-M, Guyot J-C. Compression behavior of orthorhombic paracetamol. Pharm Res. 1998;15:1122–30.
Sun CQ, Grant DJW. Influence of crystal structure on the tableting properties of sulfamerazine polymorphs. Pharm Res. 2001;18:274–80.
Feng YS, Grant DJW, Sun CC. Influence of crystal structure on the tableting properties of n-alkyl 4-hydroxybenzoate esters (Parabens). J Pharm Sci. 2007;96:3324–33.
Otsuka M, Nakanishi M, Matsuda Y. Effects of crystalline form on the tableting compression mechanism of phenobarbital polymorphs. Drug Dev Ind Pharm. 1999;25:205–15.
Jaffe J, Foss NE. Compression of crystalline substances. J Am Pharm Assoc. 1959;48:26–9.
Sun C, Grant D. Improved tableting properties of p-hydroxybenzoic acid by water of crystallization: a molecular insight. Pharm Res. 2004;21:382–6.
Bernstein J. Molecular crystals: pinching polymorphs. Nat Mater. 2005;4:427–8.
Hüttenrauch R, Fricke S, Zielke P. Mechanical activation of pharmaceutical systems. Pharm Res. 1985:302–306.
Brittain HG. Effects of mechanical processing on phase composition. J Pharm Sci. 2002;91:1573–80.
Chan HK, Doelker E. Polymorphic transformation of some drugs under compression. Drug Dev Ind Pharm. 1985;11:315–32.
Kala H, Haack U, Wenzel U, Zessin G, Pollandt P. Crystallographic behavior of carbamazepine under compression. Pharmazie. 1987;42:524–7.
Kala H, Traue J, Haack U, Moldenhauer H, Kedvessy G, Selmeczi B. Time-dependence of polymorphic changes of sulfanilamide during tablet compression. Pharmazie. 1982;37:674–5.
Ibrahim HG, Pisano F, Bruno A. Polymorphism of phenylbutazone: properties and compressional behavior of crystals. J Pharm Sci. 1977;66:669–73.
Ghan GA, Lalla JK. Effect of compressional forces on piroxicam polymorphs. J Pharm Pharmacol. 1992;44:678–81.
Kala H, Moldenhauer H, Giese R, Kedvessy G, Selmeczi B, Pintye-Hodi K. Polymorphism of sulfathiazole and its crystallographic behavior under compression pressure. Pharmazie. 1981;36:833–8.
Boldyreva E. High-pressure polymorphs of molecular solids: when are they formed, and when are they not? some examples of the role of kinetic control. Cryst Growth Des. 2007;7:1662–8.
Wildfong PL, Morris KR, Anderson CA, Short SM. Demonstration of a shear-based solid-state phase transformation in a small molecular organic system: chlorpropamide. J Pharm Sci. 2007;96:1100–13.
Okumura T, Ishida M, Takayama K, Otsuka M. Polymorphic transformation of indomethacin under high pressures. J Pharm Sci. 2006;95:689–700.
Otsuka M, Matsumoto T, Kaneniwa N. Effects of the mechanical energy of multi-tableting compression on the polymorphic transformations of chlorpropamide. J Pharm Pharmacol. 1989;41:665–9.
Koivisto M, Heinanen P, Tanninen VP, Lehto VP. Depth profiling of compression-induced disorders and polymorphic transition on tablet surfaces with grazing incidence X-ray diffraction. Pharm Res. 2006;23:813–20.
Chawla G, Bansal AK. Effect of processing on celecoxib and its solvates. Pharm Dev Technol. 2004;9:419–33.
Lefebvre C, Guyot-Hermann AM, Draguet-Brughmans M, Bouche R, Guyot JC. Polymorphic transitions of carbamazepine during grinding and compression. Drug Dev Ind Pharm. 1986;12:1913–27.
Wikström H, Marsac PJ, Taylor LS. In-line monitoring of hydrate formation during wet granulation using Raman spectroscopy. J Pharm Sci. 2005;94:209–19.
Romer M, Heinamaki J, Miroshnyk I, Sandler N, Rantanen J, Yliruusi J. Phase transformations of erythromycin A dihydrate during pelletisation and drying. Eur J Pharm Biopharm. 2007;67:246–52.
Otsuka M, Kanbniwa N, Otsuka K, Kawakami K, Umezawa O. Effect of tableting pressure and geometrical factor of tablet on dehydration kinetics of theophyline monohydrate tablets. Drug Dev Ind Pharm. 1993;19:541–57.
Suihko E, Lehto V-P, Ketolainen J, Laine E, Paronen P. Dynamic solid-state and tableting properties of four theophylline forms. Int J Pharm. 2001;217:225–36.
Ketolainen J, Poso A, Viitasaari V, Gynther J, Pirttimäki J, Laine E, et al. Changes in solid-state structure of cyclophosphamide monohydrate induced by mechanical treatment and storage. Pharm Res. 1995;12:299–304.
Brussel BAV, Hosson JTMD. Glancing angle x-ray diffraction: a different approach. Appl Phys Lett. 1994;64:1585–7.
Debnath S, Predecki P, Suryanarayanan R. Use of glancing angle X-ray powder diffractometry to depth-profile phase transformations during dissolution of indomethacin and theophylline tablets. Pharm Res. 2004;21:149–59.
Liu J, Saw RE, Kiang YH. Calculation of effective penetration depth in X-ray diffraction for pharmaceutical solids. J Pharm Sci. 2010;99:3807–14.
Pienaar EW, Caira MR, Lötter AP. Polymorphs of nitrofurantoin. 2. preparation and X-Ray crystal-structures of two anhydrous forms of nitrofurantoin. J Crystallogr Spectrosc Res. 1993;23:785–90.
Koradia V, de Diego HL, Frydenvang K, Ringkjøbing-Elema M, Müllertz A, Bond AD, et al. Solid forms of amlodipine besylate: physico-chemical, structural and thermodynamic characterization. Cryst Growth Des. 2010;10:5279–90.
Parratt LG. Surface studies of solids by total reflection of X-rays. Phys Rev. 1954;95:359–69.
Cullity BD. Elements of x-ray diffraction. Reading: Addison-Wesley; 1978.
Roisnel T, Rodriguez-Carvajal J. WinPLOTR: a windows tool for powder diffraction pattern analysis. Materials Science Forum, Proceedings of the Seventh European Powder Diffraction Conference (EPDIC 7). 378–3:118–123 (2000).
Caira MR, Pienaar EW, Lötter AP. Polymorphism and pseudopolymorphism of the antibacterial nitrofurantoin. Mol Cryst Liq Cryst. 1996;279:241–64.
Koradia V, de Diego HL, Elema MR, Rantanen J. Integrated approach to study the dehydration kinetics of nitrofurantoin monohydrate. J Pharm Sci. 2010;99:3966–76.
Phadnis NV, Suryanarayanan R. Polymorphism in anhydrous theophylline—implications on the dissolution rate of theophylline tablets. J Pharm Sci. 1997;86:1256–63.
Debnath S, Suryanarayanan R. Influence of processing-induced phase transformations on the dissolution of theophylline tablets. AAPS PharmSciTech. 2004;5:E8.
Busignies V, Leclerc B, Porion P, Evesque P, Couarraze G, Tchoreloff P. Quantitative measurements of localized density variations in cylindrical tablets using X-ray microtomography. Eur J Pharm Biopharm. 2006;64:38–50.
Eiliazadeh B, Briscoe BJ, Sheng Y, Pitt K. Investigating density distributions for tablets of different geometry during the compaction of pharmaceuticals. Part Sci Technol. 2003;21:303–16.
Hüttenrauch R, Fricke S. Mechano-chemical decomposition of drugs through galenic processes. Int J Pharm. 1979;3:289–90.
Karjalainen M, Airaksinen S, Rantanen J, Aaltonen J, Yliruusi J. Characterization of polymorphic solid-state changes using variable temperature X-ray powder diffraction. J Pharm Biomed Anal. 2005;39:27–32.
ACKNOWLEDGMENTS & DISCLOSURES
Vishal Koradia is thankful to the Drug Research Academy (Copenhagen, Denmark) and H. Lundbeck A/S (Copenhagen, Denmark) for financial support. Matrix Laboratories Limited is thanked for providing amlodipine besylate sample.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Koradia, V., Tenho, M., Lopez de Diego, H. et al. Investigation of Solid Phase Composition on Tablet Surfaces by Grazing Incidence X-ray Diffraction. Pharm Res 29, 134–144 (2012). https://doi.org/10.1007/s11095-011-0520-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-011-0520-8