Abstract
Purpose
The present study examined the underlying mechanism by which 4-hydroxyacetophenone (4-HA), a bioactive compound found in several medicinal herbs, exerts its potent stimulatory effects on hepatic bile secretion.
Methods
Bile flow, and biliary excretion of 4-HA, its metabolites, and inorganic electrolytes was examined in both normal Wistar rats and in TR- Wistar rats that have a congenital defect in the multidrug resistance-associated protein-2, Mrp2/Abcc2. The effects of 4-HA were also examined in animals treated with buthionine sulfoximine to decrease hepatic glutathione (GSH) levels.
Results
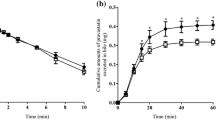
In normal rats, 4-HA dramatically increased bile flow rate, whereas it failed to exert a choleretic effect in TR- rats. This choleresis was not explained by increased biliary output of Na+, K+, Cl− or HCO3 −, or by increased biliary GSH excretion. Depletion of hepatic GSH with buthionine sulfoximine had no effect on the 4-HA-induced choleresis. HPLC analysis revealed that a single major compound was present in bile, namely.4-hydroxyacetophenone-4-O-β-glucuronide, and that the parent compound was not detected in bile. Biliary excretion of the glucuronide was directly correlated with the increases in bile flow. In contrast to normal rats, this 4-HA metabolite was not present in bile of TR− rats.
Conclusions
These results demonstrate that the major biliary metabolite of 4-HA in rats is the 4-O-β-glucuronide, a compound that is secreted into bile at high concentrations, and may thus account in large part for the choleretic effects of 4-HA. Transport of this metabolite across the canalicular membrane into bile requires expression of the Mrp2 transport protein.







Similar content being viewed by others
Abbreviations
- BADF:
-
bile acid dependent flow
- BAIF:
-
bile acid independent flow
- BSO:
-
buthionine sulfoximine
- GSH:
-
reduced glutathione
- Mrp2:
-
multidrug resistance-associated protein-2
- 4-HA:
-
4-hydroxyacetophenone
References
M. Trauner, M. Wagner, P. Fickert, and G. Zallner. Molecular regulation of hepatobiliary transport systems: Clinical implications for understanding and treating cholestasis. J. Clin. Gastroenterol.39:S111-S124 (2005).
N. Ballatori and A. T. Truong. Relation between biliary glutathione excretion and bile acid-independent bile flow. Am. J. Physiol.256:G22–G30 (1989).
N. Ballatori and A. T. Truong. Glutathione as a primary osmotic driving force in hepatic bile formation. Am. J. Physiol.263:G617–G624 (1992).
M. H. Nathanson and J. L. Boyer. Mechanism and regulation of bile secretion. Hepatology14:551–556 (1991).
P. Calhoun, K. B. Brown, R. Strunk, D. A. Krusch, M. Scheld, and J. B. Hanks. Experimental studies of biliary excretion of piperacillin. Ann. Surg.205:420–427 (1987).
J. Chenderovitch, A. Raizman, and R. Infante. Mechanism of ethacrynic acid-induced choleresis in rat. Am. J. Physiol.229:1180–1187 (1975).
J. Gonzalez, C. Fernandez, E. Marino, A. Morales, and R. Jimenez. Biliary excretion and choleretic effect of cefmetazole in rats. Antimicrob. Agents Chemother.33:1970–1974 (1989).
K. Ito, T. Koresawa, K. Nakano, and T. Horie. Mrp2 is involved in benzylpenicillin-induced choleresis. Am. J. Gastroint. Liv. Physiol.287:42–49 (2004).
P. Piyachaturawat, N. Chai-ngam, A. Chuncharunee, P. Komaratat, and A. Suksamrarn. Choleretic activity of phloracetophenone in rats: Structure-function studies using acetophenone analogues. Eur. J. Pharmacol.387:221–227 (2000).
Y. Shao, Y. L. Li, and B. N. Zhou. Phenolic and triterpenoid glycosides from Aster batangensis. Phytochemistry41:1593–1598 (1996).
E. Hideo, K. Murakami, T. Yogoh, H. Ishikawa, Y. Fukuyama, and H. Tanaka. Anti-oxidative compounds in Barley tea. Biosci. Biotechnol. Biochem.68:2616–2618 (2004).
I. Okuno, K. Uchida, M. Nakamura, and K. Sakurawi. Studies on choleretic constituents in Artemisia capillaris THUNB. Chem. Pharm. Bull. 36:769–775 (1988).
L. A. Turnberg and A. Anthony-Mote. The quantitative determination of bile salt using thin layer chromatography and 3α-hydroxysteroid dehydrogenase. Clin. Chim. Acta24:253–259 (1969).
F. Tietze. Enzymatic method for quantitative determination of nanogram amounts of total and oxidized glutathione: Applications to mammalian blood and other tissues. Anal. Biochem.27:502–522 (1969).
O. W. Griffith. Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinylpyridine. Anal. Biochem.106:207–212 (1980).
D. Alvaro, A. Mennone, and J. L. Boyer. Effect of ursodeoxycholic acid on intracellular pH regulation in isolated rat bile duct epithelial cells. Am. J. Physiol.: Gastrointest. Liver Physiol.266:G783–G791 (1993).
N. Ballatori, C. L. Hammond, J. B. Cunningham, S. M. Krance, and R. Marchan. Molecular mechanisms of reduced glutathione transport: Role of the MRP/CFTR/ABCC and OATP/SLC21A families of membrane proteins. Toxicol. Appl. Pharmacol.204:238–255 (2005).
N. Vrchotova, J. Triska, O. Urban, and L. Peknic. Variability of catechin and 4-hydroxyacetophenone distribution in Norway spruce needles in relation to their position, age, and growing conditions. Environ. Pollut.131:55–59 (2004).
M. Shimizu and I. B. Weinstein. Modulation of signal transduction by tea catechins and related phytochemicals. Mutat. Res.591(1–2):147–160 (2005).
Acknowledgments
We thank Mr. Sirichai Kositarat (Central Instrument Facility, Faculty of Science, Mahidol University) for his technical assistance with HPLC analysis of 4-hydroxyacetophenone-4-O-β-glucuronide in bile samples of normal Wistar and TR− rats.
This study was supported by grants from the Royal Golden Jubilee program (to CM), the Thailand Research Fund, a research team strengthening grant from BIOTEC, and grants DK48823 and ES01247 from the USA National Institutes of Health (to NB).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mahagita, C., Tanphichai, K., Suksamrarn, A. et al. 4-Hydroxyacetophenone-Induced Choleresis in Rats is Mediated by the Mrp2-Dependent Biliary Secretion of Its Glucuronide Conjugate. Pharm Res 23, 2603–2610 (2006). https://doi.org/10.1007/s11095-006-9097-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-006-9097-z