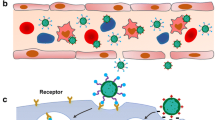
No Heading
Polymeric gene delivery systems have been developed as an alternative for viral gene delivery systems to overcome the problems in the use of viral gene carriers. Polymeric carriers have many advantages as gene carriers such as low cytotoxicity, low immunogenicity, moderate transfection efficiency, no size-limit, low cost, and reproducibility. In the efforts to develop safe and efficient polymeric gene carriers, polyethylene glycol (PEG) has widely been used because of its excellent characteristics. PEG-conjugated copolymers have advantages for gene delivery: 1) The PEG-conjugated copolymers show low cytotoxicity to cells in vitro and in vivo, 2) PEG increases water-solubility of the polymer/DNA complex, 3) PEG reduces the interaction of the polymer/DNA complex with serum proteins and increases circulation time of the complex, 4) PEG can be used as a spacer between a targeting ligand and a cationic polymer. A targeting ligand at the end of a PEG chain is not disturbed by the interaction of a cationic polymer with plasmid DNA, and the PEG spacer increases the accessibility of the ligand to its receptor. In this review, PEG copolymers as gene carriers are introduced, and their characteristics are discussed.
Similar content being viewed by others
References
1. M. L. Edelstein, M. R. Abedi, J. Wixon, and R. M. Edelstein. Gene therapy clinical trials worldwide 1989–2004—an overview. J. Gene Med. 6:597–602 (2004).
2. M. Lee and S. W. Kim. Polymeric gene carriers. Pharm. News 9:407–415 (2002).
3. G. Y. Wu and C. H. Wu. Receptor-mediated in vitro gene transformation by a soluble DNA carrier system. J. Biol. Chem. 262:4429–4432 (1987).
4. G. Y. Wu and C. H. Wu. Evidence for targeted gene delivery to Hep G2 hepatoma cells in vitro. Biochemistry 27:887–892 (1988).
5. G. Y. Wu, J. M. Wilson, F. Shalaby, M. Grossman, D. A. Shafritz, and C. H. Wu. Receptor-mediated gene delivery in vivo. Partial correction of genetic analbuminemia in Nagase rats. J. Biol. Chem. 266:14338–14342 (1991).
6. S. Zalipsky and J. M. Harris. Introduction to chemistry and biological applications of poly(ethylene glycol). In J. M. Harris and S. Zalipsky (eds.), Poly(ehtylene glycol): Chemistry and Biological Applications, Vol. 680. ACS Symposium Series. American Chemical Society, Washington, DC, 1997, pp. 1–13.
7. E. Wagner, M. Cotton, R. Foisner, and M. L. Birnstiel. Transferrin-polycation-DNA complexes: the effect of polycations on the structure of the complex and DNA delivery to cells. Proc. Natl. Acad. Sci. USA 88:4255–4259 (1991).
8. M. Lee, J. Rentz, S. Han, D. A. Bull, and S. W. Kim. Water soluble lipopolymer as an efficient carrier for gene delivery to myocardium. Gene Ther. 10:585–593 (2003).
9. J. H. Jeong and T. G. Park. Poly(L-lysine)-g-poly(D, L-lactic-co-glycolic acid) micelles for low cytotoxic biodegradable gene carriers. J. Control. Rel. 82:159–166 (2002).
10. D. Y. Kwoh, C. C. Coffin, C. P. Lollo, J. Jovenal, M. G. Banaszczyk, P. Mullen, A. Phillips, A. Amini, J. Fabrycki, R. M. Bartholomew, S. W. Brostoff, and D. J. Carlo. Stabilization of poly-L-lysine/DNA polyplexes for in vivo gene delivery to the liver. Biochim. Biophys. Acta 1444:171–190 (1999).
11. M. Ogris, S. Brunner, S. Schuller, R. Kircheis, and E. Wagner. PEGylated DNA/transferrin-PEI complexes: reduced interaction with blood components, extended circulation in blood and potential for systemic gene delivery. Gene Ther. 6:595–605 (1999).
12. P. R. Dash, V. Toncheva, E. Schacht, and L. W. Seymour. Synthetic polymers for vectorial delivery of DNA: characterisation of polymer-DNA complexes by photon correlation spectroscopy and stability to nuclease degradation and disruption by polyanions in vitro. J. Control. Rel. 48:269–276 (1997).
13. A. V. Kabanov and V. A. Kabanov. DNA complexes with polycations for the delivery of genetic material into cells. Bioconjug. Chem. 6:7–20 (1995).
14. M. A. Wolfert, E. H. Schacht, V. Toncheva, K. Ulbrich, O. Nazarova, and L. W. Seymour. Characterization of vectors for gene therapy formed by self-assembly of DNA with synthetic block copolymers. Hum. Gene Ther. 7:2123–2133 (1996).
15. S. Katayose and K. Kataoka. Water-soluble polyion complex associates of DNA and poly(ethylene glycol)-poly(L-lysine) block copolymer. Bioconjug. Chem. 8:702–707 (1997).
16. S. Katayose and K. Kataoka. Remarkable increase in nuclease resistance of plasmid DNA through supramolecular assembly with poly(ethylene glycol)-poly(L-lysine) block copolymer. J. Pharm. Sci. 87:160–163 (1998).
17. A. Harada, H. Togawa, and K. Kataoka. Physicochemical properties and nuclease resistance of antisense-oligodeoxynucleotides entrapped in the core of polyion complex micelles composed of poly(ethylene glycol)-poly(L-lysine) block copolymers. Eur. J. Pharm. Sci. 13:35–42 (2001).
18. M. Harada-Shiba, K. Yamauchi, A. Harada, I. Takamisawa, K. Shimokado, and K. Kataoka. Polyion complex micelles as vectors in gene therapy—pharmacokinetics and in vivo gene transfer. Gene Ther. 9:407–414 (2002).
19. J. S. Choi, E. J. Lee, Y. H. Choi, Y. J. Jeong, and J. S. Park. Poly-(ethylene glycol)-block-poly(L-lysine) dendrimer: novel linear polymer/dendrimer block copolymer forming a spherical water-soluble polyionic complex with DNA. Bioconjug. Chem. 10:62–65 (1999).
20. J. S. Choi, D. K. Joo, C. H. Kim, K. Kim, and J. S. Park. Synthesis of a barbell-like triblock, poly(L-lysine) dendrimer-block-poly-(ethylene glycol)-block-poly(L-lysine) dendrimer, and its self-assembly with plasmid DNA. J. Am. Chem. Soc. 122:474–480 (2000).
21. M. Bikram, C. H. Ahn, S. Y. Chae, M. Lee, J. W. Yockman, and S. W. Kim. Biodegradable poly(ethylene glycol)-co-poly(L-lysine)-g-histidine multiblock copolymers for nonviral gene delivery. Macromolecules 37:1903–1916 (2004).
22. Y. H. Choi, F. Liu, J. S. Kim, Y. K. Choi, J. S. Park, and S. W. Kim. Polyethylene glycol-grafted poly-L-lysine as polymeric gene carrier. J. Control. Rel. 54:39–48 (1998).
23. M. Lee, S. O. Han, K. S. Ko, J. J. Koh, J. S. Park, J. W. Yoon, and S. W. Kim. Repression of GAD autoantigen expression in pancreas beta-Cells by delivery of antisense plasmid/PEG-g-PLL complex. Mol. Ther. 4:339–346 (2001).
24. J. W. Yoon, C. S. Yoon, H. W. Lim, Q. Q. Huang, Y. Kang, K. H. Pyun, K. Hirasawa, R. S. Sherwin, and H. S. Jun. Control of autoimmune diabetes in NOD mice by GAD expression or suppression in beta cells. Science 284:1183–1187 (1999).
25. V. Toncheva, M. A. Wolfert, P. R. Dash, D. Oupicky, K. Ulbrich, L. W. Seymour, and E. H. Schacht. Novel vectors for gene delivery formed by self-assembly of DNA with poly(L-lysine) grafted with hydrophilic polymers. Biochim. Biophys. Acta 1380:354–368 (1998).
26. O. Boussif. F. Lezoualc’h, M. A. Zanta, M. D. Mergny, D. Scherman, B. Demeneix, and J. P. Behr. A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo: polyethylenimine. Proc. Natl. Acad. Sci. USA 92:7297–7301 (1995).
27. O. Boussif, M. A. Zanta, and J. P. Behr. Optimized galenics improve in vitro gene transfer with cationic molecules up to 1000-fold. Gene Ther. 3:1074–1080 (1996).
28. D. D. Dunlap, A. Maggi, M. R. Soria, and L. Monaco. Nanoscopic structure of DNA condensed for gene delivery. Nucleic Acids Res. 25:3095–3101 (1997).
29. W. T. Godbey, K. K. Wu, and A. G. Mikos. Poly(ethylenimine) and its role in gene delivery. J. Control. Rel. 60:149–160 (1999).
30. H. Petersen, P. M. Fechner, A. L. Martin, K. Kunath, S. Stolnik, C. J. Roberts, D. Fischer, M. C. Davies, and T. Kissel. Polyethylenimine-graft-poly(ethylene glycol) copolymers: influence of copolymer block structure on DNA complexation and biological activities as gene delivery system. Bioconjug. Chem. 13:845–854 (2002).
31. L. Shi, G. P. Tang, S. J. Gao, Y. X. Ma, B. H. Liu, Y. Li, J. M. Zeng, Y. K. Ng, K. W. Leong, and S. Wang. Repeated intrathecal administration of plasmid DNA complexed with polyethylene glycol-grafted polyethylenimine led to prolonged transgene expression in the spinal cord. Gene Ther. 10:1179–1188 (2003).
32. H. Petersen, K. Kunath, A. L. Martin, S. Stolnik, C. J. Roberts, M. C. Davies, and T. Kissel. Star-shaped poly(ethylene glycol)-block-polyethylenimine copolymers enhance DNA condensation of low molecular weight polyethylenimines. Biomacromolecules 3:926–936 (2002).
33. A. Kichler, M. Chillon, C. Leborgne, O. Danos, and B. Frisch. Intranasal gene delivery with a polyethylenimine-PEG conjugate. J. Control. Rel. 81:379–388 (2002).
34. C. H. Ahn, S. Y. Chae, Y. H. Bae, and S. W. Kim. Biodegradable poly(ethylenimine) for plasmid DNA delivery. J. Control. Rel. 80:273–282 (2002).
35. R. Kircheis, S. Schuller, S. Brunner, M. Ogris, K. H. Heider, W. Zauner, and E. Wagner. Polycation-based DNA complexes for tumor-targeted gene delivery in vivo. J. Gene Med. 1:111–120 (1999).
36. R. Kircheis, T. Blessing, S. Brunner, L. Wightman, and E. Wagner. Tumor targeting with surface-shielded ligand-polycation DNA complexes. J. Control. Rel. 72:165–170 (2001).
37. C. Rudolph, U. Schillinger, C. Plank, A. Gessner, P. Nicklaus, R. Muller, and J. Rosenecker. Nonviral gene delivery to the lung with copolymer-protected and transferrin-modified polyethylenimine. Biochim. Biophys. Acta 1573:75–83 (2002).
38. D. Oupicky, M. Ogris, K. A. Howard, P. R. Dash, K. Ulbrich, and L. W. Seymour. Importance of lateral and steric stabilization of polyelectrolyte gene delivery vectors for extended systemic circulation. Mol. Ther. 5:463–472 (2002).
39. W. Suh, S. O. Han, L. Yu, and S. W. Kim. An angiogenic, endothelial-cell-targeted polymeric gene carrier. Mol. Ther. 6:664–672 (2002).
40. P. C. Brooks, R. A. Clark, and D. A. Cheresh. Requirement of vascular integrin alpha v beta 3 for angiogenesis. Science 264:569–571 (1994).
41. D. A. Sipkins, D. A. Cheresh, M. R. Kazemi, L. M. Nevin, M. D. Bednarski, and K. C. Li. Detection of tumor angiogenesis in vivo by alphaVbeta3-targeted magnetic resonance imaging. Nat. Med. 4:623–626 (1998).
42. G. Gasparini, P. C. Brooks, E. Biganzoli, P. B. Vermeulen, E. Bonoldi, L. Y. Dirix, G. Ranieri, R. Miceli, and D. A. Cheresh. Vascular integrin alpha(v)beta3: a new prognostic indicator in breast cancer. Clin. Cancer Res. 4:2625–2634 (1998).
43. A. Erdreich-Epstein, H. Shimada, S. Groshen, M. Liu, L. S. Metelitsa, K. S. Kim, M. F. Stins, R. C. Seeger, and D. L. Durden. Integrins alpha(v)beta3 and alpha(v)beta5 are expressed by endothelium of high-risk neuroblastoma and their inhibition is associated with increased endogenous ceramide. Cancer Res. 60:712–721 (2000).
44. K. Kunath, T. Merdan, O. Hegener, H. Haberlein, and T. Kissel. Integrin targeting using RGD-PEI conjugates for in vitro gene transfer. J. Gene Med. 5:588–599 (2003).
45. P. Midoux, C. Mendes, A. Legrand, J. Raimond, R. Mayer, M. Monsigny, and A. C. Roche. Specific gene transfer mediated by lactosylated poly-L-lysine into hepatoma cells. Nucleic Acids Res. 21:871–878 (1993).
46. M. Hashida, S. Takemura, M. Nishikawa, and Y. Takakura. Targeted delivery of plasmid DNA complexed with galactosylated poly(L-lysine). J. Control. Rel. 53:301–310 (1998).
47. M. Nishikawa, S. Takemura, Y. Takakura, and M. Hashida. Targeted delivery of plasmid DNA to hepatocytes in vivo: optimization of the pharmacokinetics of plasmid DNA/galactosylated poly(L-lysine) complexes by controlling their physicochemical properties. J. Pharmacol. Exp. Ther. 287:408–415 (1998).
48. R. I. Mahato, S. Takemura, K. Akamatsu, M. Nishikawa, Y. Takakura, and M. Hashida. Physicochemical and disposition characteristics of antisense oligonucleotides complexed with glycosylated poly(L-lysine). Biochem. Pharmacol. 53:887–895 (1997).
49. Y. H. Choi, F. Liu, J. S. Park, and S. W. Kim. Lactose-poly(ethylene glycol)-grafted poly-L-lysine as hepatoma cell-targeted gene carrier. Bioconjug. Chem. 9:708–718 (1998).
50. Y. H. Choi, F. Liu, J. S. Choi, S. W. Kim, and J. S. Park. Characterization of a targeted gene carrier, lactose-polyethylene glycol-grafted poly-L-lysine and its complex with plasmid DNA. Hum. Gene Ther. 10:2657–2665 (1999).
51. T. Bettinger, J. S. Remy, and P. Erbacher. Size reduction of galactosylated PEI/DNA complexes improves lectin-mediated gene transfer into hepatocytes. Bioconjug. Chem. 10:558–561 (1999).
52. K. Sagara and S. W. Kim. A new synthesis of galactose-poly(ethylene glycol)-polyethylenimine for gene delivery to hepatocytes. J. Control. Rel. 79:271–281 (2002).
53. R. J. Havel. Receptor and non-receptor mediated uptake of chylomicron remnants by the liver. Atherosclerosis 141:S1–S7 (1998).
54. I. L. Shih, R. S. Lees, M. Y. Chang, and A. M. Lees. Focal accumulation of an apolipoprotein B-based synthetic oligopeptide in the healing rabbit arterial wall. Proc. Natl. Acad. Sci. USA 87:1436–1440 (1990).
55. M. Lougheed, E. D. Moore, D. R. Scriven, and U. P. Steinbrecher. Uptake of oxidized LDL by macrophages differs from that of acetyl LDL and leads to expansion of an acidic endolysosomal compartment. Arterioscler. Thromb. Vasc. Biol. 19:1881–1890 (1999).
56. J. W. Nah, L. Yu, S. O. Han, C. H. Ahn, and S. W. Kim. Artery wall binding peptide-poly(ethylene glycol)-grafted-poly(L-lysine)-based gene delivery to artery wall cells. J. Control. Rel. 78:273–284 (2002).
57. J. J. Turek, C. P. Leamon, and P. S. Low. Endocytosis of folate-protein conjugates: ultrastructural localization in KB cells. J. Cell Sci. 106:423–430 (1993).
58. S. Wang and P. S. Low. Folate-mediated targeting of antineoplastic drugs, imaging agents, and nucleic acids to cancer cells. J. Control. Rel. 53:39–48 (1998).
59. K. A. Mislick, J. D. Baldeschwieler, J. F. Kayyem, and T. J. Meade. Transfection of folate-polylysine DNA complexes: evidence for lysosomal delivery. Bioconjug. Chem. 6:512–515 (1995).
60. C. M. Ward, M. Pechar, D. Oupicky, K. Ulbrich, and L. W. Seymour. Modification of pLL/DNA complexes with a multivalent hydrophilic polymer permits folate-mediated targeting in vitro and prolonged plasma circulation in vivo. J. Gene Med. 4:536–547 (2002).
61. C. P. Leamon, D. Weigl, and R. W. Hendren. Folate copolymer-mediated transfection of cultured cells. Bioconjug. Chem. 10:947–957 (1999).
62. J. M. Benns, A. Maheshwari, D. Y. Furgeson, R. I. Mahato, and S. W. Kim. Folate-PEG-folate-graft-polyethylenimine-based gene delivery. J. Drug Target. 9:123–139 (2001).
63. J. M. Benns, R. I. Mahato, and S. W. Kim. Optimization of factors influencing the transfection efficiency of folate-PEG-folate-graft-polyethylenimine. J. Control. Rel. 79:255–269 (2002).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lee, M., Kim, S. Polyethylene Glycol-Conjugated Copolymers for Plasmid DNA Delivery. Pharm Res 22, 1–10 (2005). https://doi.org/10.1007/s11095-004-9003-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-004-9003-5