Abstract
The thermodynamic potential for the abiotic synthesis of the five common nucleobases (adenine, cytosine, guanine, thymine, and uracil) and two monosaccharides (ribose and deoxyribose) from formaldehyde and hydrogen cyanide has been quantified under temperature, pressure, and bulk composition conditions that are representative of hydrothermal systems. The activities of the precursor molecules (formaldehyde and hydrogen cyanide) required to evaluate the thermodynamics of biomolecule synthesis were computed using the concentrations of aqueous N2, CO, CO2 and H2 reported in the modern Rainbow hydrothermal system. The concentrations of precursor molecules that can be synthesized are strongly dependent on temperature with larger concentrations prevailing at lower temperatures. Similarly, the thermodynamic drive to synthesize nucleobases, ribose and deoxyribose varies considerably as a function of temperature: all of the biomolecules considered in this study are thermodynamically favored to be synthesized throughout the temperature range from 0°C to between 150°C and 250°C, depending on the biomolecule. Furthermore, activity diagrams have been generated to illustrate that activities in the range of 10−2– 10−6 for nucleobases, ribose and deoxyribose can be in equilibrium with a range of precursor molecule activities at 150°C and 500 bars. The results presented here support the notion that hydrothermal systems could have played a fundamental role in the origin of life, and can be used to plan and constrain experimental investigation of the abiotic synthesis of nucleic-acid related biomolecules.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In recent years many theoretical and experimental studies have tested the hypothesis that ancient deep sea hydrothermal systems provided an environment conducive to the abiotic synthesis of biomolecules that are essential for the emergence of life (Bock and Goode 1996; Hazen et al. 2002; Holm 1992; Holm and Andersson 1995, 2005; Holm et al. 2006; McCollom and Seewald 2007; Nisbet and Fowler 1996; Shock 1990, 1992b, 1996; Shock et al. 2000; Simoneit 2004; Woese 1998; Woese et al. 1990). As a result, abiotic synthesis studies involving amino acids (Amend and Shock 1998, 2000; Hennet et al. 1992; Marshall 1994; Shock and Schulte 1990), lipid-like compounds (McCollom et al. 1999; Rushdi and Simoneit 2001), aldehydes (Shock and Schulte 1993), carboxylic acids (McCollom and Seewald 2001, 2003a, b), alcohols, ketones (Shock and Schulte 1995, 1998), polycyclic, alkylated and hydroxylated aromatic hydrocarbons (McCollom 2003; Williams et al. 2005; Zolotov and Shock 1999), thiols (Rushdi and Simoneit 2005; Schulte and Rogers 2004) and other organic compounds (Foustoukos and Seyfried 2004; McCollom and Simoneit 1999; Rushdi and Simoneit 2004; Shock and McKinnon 1993) have been carried out under hydrothermal conditions using experimental and/or computational–thermodynamic techniques. However, the common organic monomers constituting nucleic acids, that is, adenine, cytosine, guanine, cytosine, thymine, uracil, ribose, and deoxyribose, have received far less attention. A notable exception is the recent work carried out by Franiatte and coworkers in which the stability of aqueous adenine was measured at 300°C under controlled fugacities of H2, CO2, and N2 (Franiatte et al. 2008). Exploring potential extraterrestrial sources of nucleobases, Saldino and coworkers have synthesized adenine cytosine, thymine and uracil in one-pot experiments from 0.12 mM formamide (CH3NO) at 160°C in the presence of cosmic dust analogues (amorphous olivines), TiO2 and montmorillonites (Saladino et al. 2003, 2004, 2005b) (for a review of experimental abiotic nucleobase synthesis see Saladino et al. 2005a). Similarly, several other experiments, carried out using high concentrations of precursor molecules, organic solvent extractions and a variety of catalysts under unspecified oxidation states, have produced nucleobases in gas, solid and aqueous phases (Ferris et al. 1968; Hayatsu et al. 1968; Hill and Orgel 2002; Miyakawa et al. 2000; Wakamatsu et al. 1966). But because the conditions under which these experiments were performed do not reflect those of any known current or past hydrothermal systems, their relevance to natural systems is unclear. The purpose of the present study is to explore the thermodynamic potential for the abiotic synthesis of the five common nucleobases (adenine, guanine, cytosine, thymine and uracil) and two sugars (ribose & deoxyribose) that make up nucleic acids (DNA & RNA) from two precursor molecules, formaldehyde (CH2O) and hydrogen cyanide (HCN), over a range of pressures, temperatures, and bulk compositions that are characteristic of hydrothermal systems. This is accomplished by first quantifying the thermodynamic potential to synthesize the precursor molecules from N2, H2, CO2 and CO, and then calculating the energetics of biomolecular synthesis from the two precursor compounds.
Precursor Molecules
The successful experimental synthesis of organic compounds from the condensation of CH2O and HCN, usually referred to as Strecker synthesis, has lead to the hypothesis that reactions involving these precursor molecules were responsible for the abiotic synthesis of biomolecules on the early Earth (Miller 1957; Ferris et al. 1978; Schulte and Shock 1993, 1995). Oró and coworkers were among the first to contribute to this idea by synthesizing adenine, C5H5N5, from HCN (Oró 1960; Oró and Kimball 1961). In addition, it has long been established that carbohydrates of varying carbon number, such as ribose, C5H10O5, can be made abiotically from formaldehyde according to the formose reaction (Butlerov 1861). More recently, Hennet et al. (1992) synthesized several amino acids from CH2O and HCN at 150°C. The prevalence of CH2O and HCN in ancient or modern hydrothermal systems is not known, but the amount of these compounds that could have existed in prebiotic hydrothermal systems can be estimated by taking into account reactions among simple sources of N, C, H and O in these environments that can be relatively well-constrained.
Hydrogen Cyanide
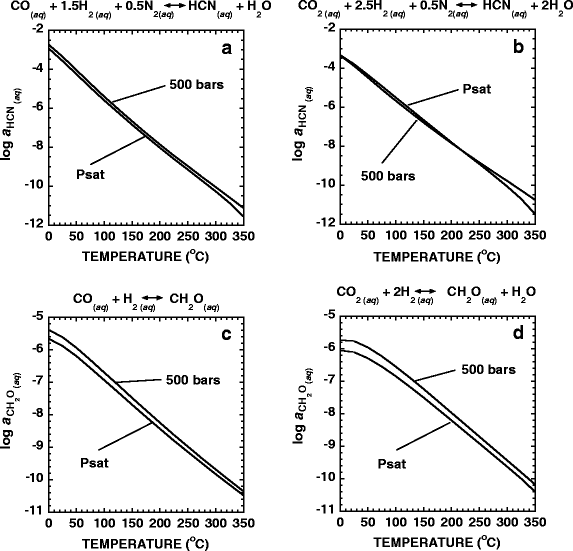
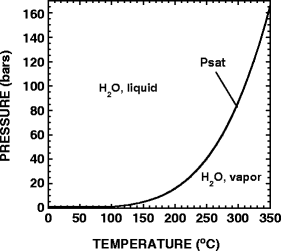
HCN in hydrothermal systems could come from reactions between H2, N2, and CO2 and CO (Schulte and Shock 1995; Shock 1992a), all of which are considered to have been common species on the early Earth (Kasting 1993). The activities of aqueous HCN in equilibrium with N2, H2 and CO and N2, H2 and CO2 from 0°C to 350°C, at pressures that are either equal to those corresponding to the liquid/vapor saturation curve for H2O (Psat) or at 500 bars are shown in Fig. 1a,b, respectively. The phase diagram for H2O shown in Fig. 2 displays the values of pressure used in the Psat calculations as a function of temperature. It can be seen in Fig. 1a,b that the activity of HCN in equilibrium with N2, H2 and CO or CO2 decreases substantially as temperature increases and that pressure has little effect on the magnitude of this variation.
Temperature-activity diagrams depicting the activities of CH2O and HCN that are in equilibrium with the reactants and products shown in the reactions above each panel. The concentrations of CO, CO2, N2, and H2 used to generate these figures are given in Table 1
The curves in Fig. 1a,b were generated by first calculating the equilibrium constant, K, for
and
Statements of the law of mass action for these reactions, e.g.,
were then rearranged to solve for the equilibrium activity of the species of interest:
Similarly, the logarithmic equivalent for Reaction (2) can be written:
where K 1 and K 2 refer to the equilibrium constants of Reactions (1) and (2), respectively. Values of K for these and the following reactions were calculated using
where R stands for the gas constant, T represents the absolute temperature and \(\Delta G_{\text{r}}^0 \) denotes the standard molal Gibbs energy of reaction. Values of \(\Delta G_{\text{r}}^0 \) were computed with the SUPCRT92 software package (Johnson et al. 1992) which relies on the revised Helgeson–Kirkham–Flowers (HKF) equations of state (Shock and Helgeson 1990; Tanger and Helgeson 1988) to calculate the thermodynamic properties of chemical reactions at elevated temperatures and pressures. The standard state adopted in the present study for aqueous species other than H2O corresponds to unit activity of the species in a hypothetical one molal solution referenced to infinite dilution at any pressure and temperature (see LaRowe and Helgeson 2007). The activity of liquid H2O was taken to be 1. The activities of N2, H2, CO and CO2 were specified by multiplying the measured concentrations of these species (Table 1) in the presently-active Rainbow hydrothermal system, located at 36°14′ N on the Mid-Atlantic Ridge at a depth of 2,300 m (Charlou and colleagues reported fluids emanating at 365°C at pH=2.8 (Charlou et al. 2002)), with activity coefficients, γ, that were computed using the HCh software package (Shvarov and Bastrakov 1999). Because the ionic strength used in the activity coefficient calculations is that of modern seawater, values of γ for neutral species were generally slightly less than one (∼0.975). This approach was used principally because the composition of ancient hydrothermal systems is unknown, and, at the very least, the modern concentrations represent a proxy for characterizing geochemical solutions that may have prevailed in the past. In addition, this is the rare hydrothermal systems for which concentrations of CO, CO2, H2 and N2 have been reported in the literature. To specify the composition of ancient hydrothermal fluids, Schulte and Shock (1995) followed a different approach that used instead fugacities of CO, CO2, and N2 constrained from estimated ancient atmospheric concentrations of the same species made by Kasting (1993). However, a cursory examination of modern hydrothermal vent compositions (Charlou et al. 2002; Von Damm 1995) reveals that the concentrations of CO, CO2, and N2 are highly variable from one system to another and do not correlate with their concentrations in the modern atmosphere. The assumption that the concentrations of the species in ancient and modern hydrothermal fluids are similar is reasonable because the composition of hydrothermal fluids are primarily determined by their interactions with mantle-derived rocks, whose composition (i.e., oxidation state) has not changed significantly in the last four billion years (Delano 2001).
Formaldehyde
Formaldehyde, CH2O, is often cited as a common prebiotic molecule (Orgel 2004; Shapiro 1988), but the concentration of this compound on the early earth is not known. In order to quantify possible concentrations of aqueous CH2O in hydrothermal systems, as with the HCN, equilibrium activities of this species have been calculated using the H2, CO and CO2 concentrations from the Rainbow site (Fig. 1c,d). Although the equilibrium activity of CH2O follows the same qualitative trend with temperature as the activity of HCN shown in Fig. 1a,b, these calculations show that higher pressure (500 bars) favors higher equilibrium activities of CH2O relative to lower pressure conditions (Psat). However, even under the most favorable conditions of low temperatures and high pressure, the equilibrium activity of CH2O, with respect to H2 and CO and CO2, is much less than HCN and 10−5. These results reflect the conditions in one modern hydrothermal system only so if the concentrations of H2, CO and CO2 would have been higher in ancient hydrothermal systems, the activity of CH2O in equilibrium with these reactants could also have been greater. Kasting (1993) estimated that during the first several hundred million years of Earth’s history the atmosphere contained CO + CO2 equal to 10 bars, a result which suggests that the concentration of precursor carbon molecules in hydrothermal systems could have been different from those today.
The curves in Fig. 1c,d were generated following the strategy outlined above for HCN, but in accordance with
and
The equilibrium activities of HCN and CH2O shown in Fig. 1 are used in the following section to calculate the thermodynamic drive for the abiotic synthesis of nucleobases, ribose and deoxyribose.
Nucleobase, Ribose and Deoxyribose Synthesis
In this section, the thermodynamic drive to synthesize the five common nucleobases, ribose and deoxyribose is quantified by calculating the Gibbs energy of reaction for each of these compounds from the precursor molecules HCN and CH2O as a function of temperature and temperature.
Nucleobases
The nucleobases considered in this study are the common purines (adenine and guanine) and pyrimidines (cytosine, thymine, and uracil) found in DNA and RNA. Experimental attempts to synthesize these compounds abiotically have been less successful than for amino acids, especially guanine and uracil (Saladino et al. 2005a).
The overall Gibbs energy of reaction, ΔG r, for the synthesis of the five common nucleobases from aqueous HCN and CH2O are calculated for
and
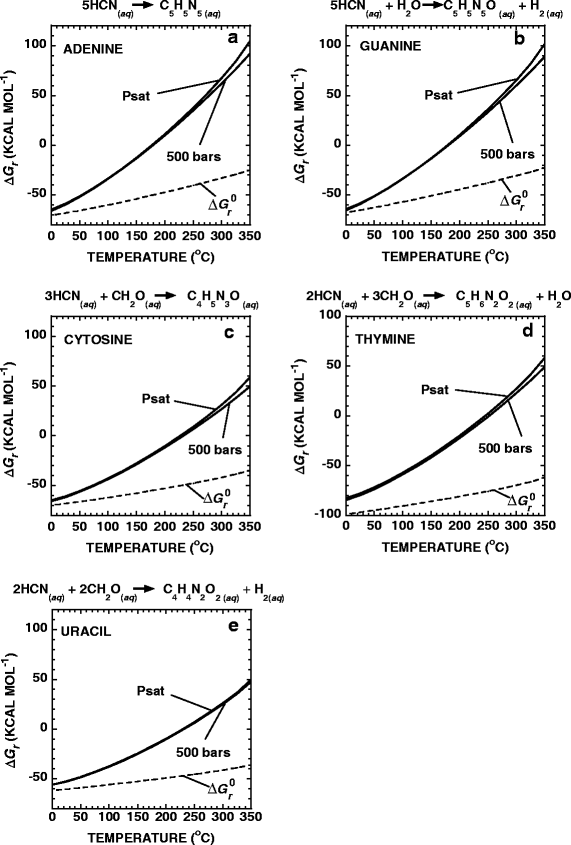
and are shown as a function of temperature at Psat and 500 bars in Fig. 3a–e. It can be seen in this figure that the values of ΔG r are negative for Reactions 9–13 from 0°C to between 175°C and 240°C, depending on the nucleobase. Above these temperatures, Reactions 9–13 are no longer favored. The small difference in values of ΔG r between the Psat and 500-bar curves indicate that pressure has little effect on these reactions. The dashed lines in Fig. 3a–e represent values of \(\Delta G_{\text{r}}^0 \) for the respective reactions. These lines are shown to illustrate the quantitative difference between the energetics of chemical reactions when the activities of the reactants and products are (ΔG r) and are not (\(\Delta G_{\text{r}}^{\text{0}} \)) taken into account.
Standard (dashed lines) and overall Gibbs energies of reaction (\(\Delta G_{\text{r}}^0 \) and ΔG r, respectively) for the synthesis of nucleobases from CH2O and HCN in accordance with the reactions shown above each panel. The activities of CH2O and HCN used to construct these diagrams are shown in Fig. 1, while those for the nucleobases were all set equal to 10−12
Values of the Gibbs energy of reaction were calculated using
where Q refers to the activity product, which is defined by
where the symbols a i and ν i denote the activity and stoichiometric reaction coefficient of the ith species, respectively. The standard molal thermodynamic properties and revised HKF equation of state parameters for the nucleobases required to calculate values of K were taken from LaRowe and Helgeson (2006). The values of a i for HCN and CH2O were taken from the results shown in Fig. 1b,d. The ΔG r curves, calculated using CO as a carbon source, are not included here because they are nearly identical to the CO2-derived calculations. The activities of H2 required to evaluate Eq. 15 for reactions 10 & 13 were taken from the Rainbow hydrothermal system (Table 1). The activities of the nucleobases were taken to be 10−12, after (McCollom and Amend 2005).
Ribose and Deoxyribose
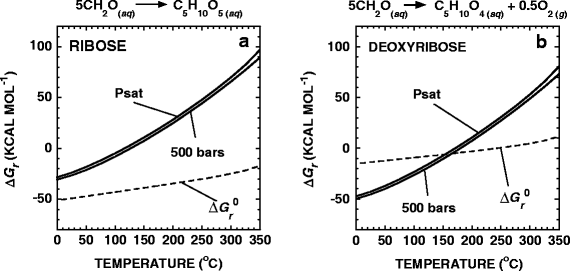
Values of ΔG r for the synthesis of aqueous ribose, C5H10O5 (aq), and deoxyribose, C5H10O4 (aq), from CO2-derived formaldehyde,
and
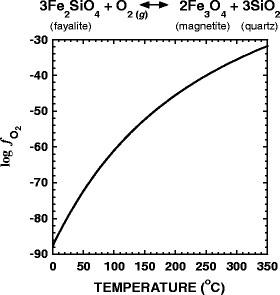
are shown as a function of temperature at Psat and 500 bars in Fig. 4a,b, respectively. For reference, the dashed lines in these figures stand for values of \(\Delta G_{\text{r}}^0 \) for the respective reactions. The overall Gibbs energy of reaction for Reactions 16 and 17 are negative from 0°C to ∼140°C and 0°C to ∼175°C, respectively. The temperatures at which these reactions become endergonic at Psat and 500 bars differ by 5°C or less. These calculations were carried out using Eqs. 14 and 15 and the thermodynamic properties of ribose and deoxyribose (Amend and Plyasunov 2001; LaRowe and Helgeson 2006). The values of a i for CH2O required to evaluate Eq. 15 were taken from the equilibrium activities of CO2-derived formaldehyde shown in Fig. 1d. The activities of ribose and deoxyribose were set equal to 10−12. The values of oxygen fugacity, \(f_{{\text{O}}_{\text{2}} } \), used to compute Q for Reaction 17 were determined by calculating the oxygen fugacity that is in equilibrium with the fayalite, magnetite, quartz (FMQ) buffer for the temperatures and pressures considered here, a common proxy for the oxidation state of submarine hydrothermal systems (Schulte and Shock 1995). These values are shown in Fig. 5.
Standard (dashed lines) and overall Gibbs energies of reaction (\(\Delta G_{\text{r}}^0 \) and ΔG r, respectively) for the synthesis of ribose and deoxyribose from CH2O in accordance with the reactions shown above each panel. The activities of CH2O used to construct these diagrams are shown in Fig. 1, while those for the sugars were all set equal to 10−12. The values of oxygen fugacity used in the calculations for deoxyribose are taken to be equal to those in equilibrium with the FMQ buffer, which is characteristic of hydrothermal systems (Schulte and Shock 1995)
The feasibility of Reaction 16 representing a likely pathway for the abiotic synthesis of ribose has been doubted (Shapiro 1988, 1995). However, due to recent progress in determining the chemical environments that promote the abiotic synthesis of ribose (Orgel 2004; Ferris 2005; Holm et al. 2006), the likelihood that Reaction 16 represents a plausible origin of this fundamental sugar has been substantiated. Initially, a number of studies showed that, under undiscriminating laboratory conditions, the formose reaction produces dozens of aldoses, ketoses, and sugar alcohols from CH2O, i.e., very little ribose (Shapiro 1988). However, it has recently been found that in the presence of Pb2+, aldopentoses are preferentially formed during the formose reaction and some of these reaction products are isomerized to ribose (Zubay 1998; Zubay and Mui 2001). Also, Ricardo et al. (2004) showed that borate minerals preferentially bind pentoses, ribose most so, thus stabilizing and potentially concentrating them over other pentoses. Furthermore, when phosphate is present, ribose-phosphate compounds, the repetitive-unit backbone of RNA, are formed and do not readily convert into other molecules (Müller et al. 1990).
Variable CH2O and HCN Activities
Because the concentrations of N2, H2, CO, and CO2 used to calculate the activities of CH2O and HCN in this study are highly variable in modern systems (Von Damm 1995) and, likely, ancient hydrothermal systems, the activities of the precursor molecules produced in them may have also been quite variable. In order to quantitatively assess the impact that variable precursor molecule concentrations would have on the production of nucleobases, ribose and deoxyribose, the activities of these species in equilibrium with varying activities of CH2O and HCN and, where relevant, H2 and O2, were calculated under temperature and pressure conditions that favor their formation.
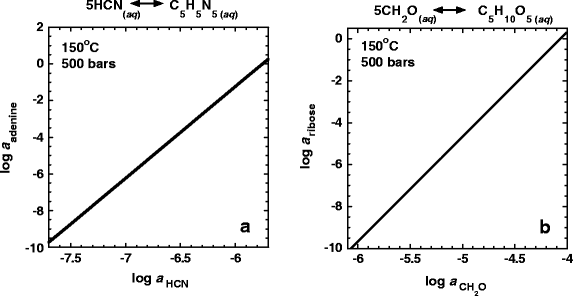
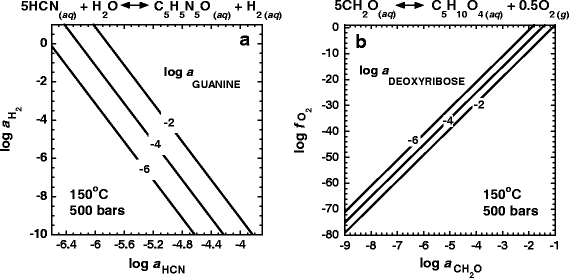
The activities of adenine and ribose in equilibrium with varying activities of HCN and CH2O, respectively, at 150°C and 500 bars are shown in Fig. 6a,b. Activities of adenine between 10−2– 10−6 are in equilibrium with those of HCN equal to from 10−6 to 10−7. Similarly, ribose can achieve equilibrium activities between 10−2– 10−6 with CH2O activities ranging from 10−4.5 to 10−5.2. The activities of the other biomolecules considered in this study depend on more than one chemical variable and are thus represented on the plane intersecting this parameter space. For example, Fig. 7a,b show contoured activities of guanine and deoxyribose as a function of the activities of HCN and CH2O, respectively, and the oxidation state. In the case of guanine, the oxidation state is described by the activity of H2, \(a_{{\text{H}}_{\text{2}} } \), while that for deoxyribose is the fugacity of oxygen. It can be seen in both Fig. 7a,b that large activities of guanine and deoxyribose (10−2– 10−6) are in equilibrium with relatively small activities of their respective precursor molecules (log a HCN = −3.8 to −6.6 in the case of guanine and log a CH2O = −1 to −9 for deoxyribose). Also, in both cases, for a given activity of precursor molecule, more reducing conditions favor higher activities of guanine and deoxyribose.
Contours of logarithms of the activities of guanine (a) and deoxyribose (b) in equilibrium with variable activities of HCN and H2 and CH2O and O2, respectively, at 150°C and 500 bars. The reaction written above each panel represents the equilibrium relationship between the species of interest and precursor molecules
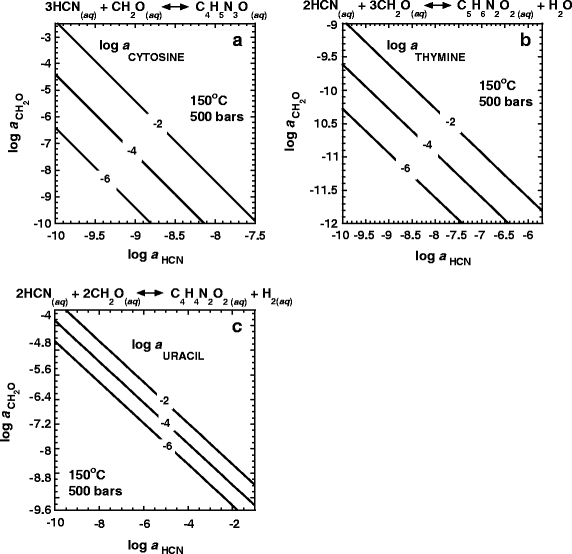
Contoured equilibrium activities of cytosine, thymine and uracil are shown as a function of the activities of CH2O and HCN in Fig. 8a–c. All three of these pyrimidines can coexist at high activities (10−2– 10−6) with relatively low activities of the precursor molecules (10−10 or lower) at 150°C and 500 bars.
Conclusions
Thermodynamic calculations have revealed that adenine, guanine, cytosine, thymine, uracil, ribose, and deoxyribose can be synthesized from the precursor molecules CH2O and HCN under temperatures, pressures, and fluid compositions that are characteristic of hydrothermal systems. However, nucleobase and sugar synthesis is thermodynamically favored only at the lower end of the temperature range considered here. This finding corroborates previous studies which have suggested that organic synthesis in submarine hydrothermal systems most likely did not occur in black smoker vent sites where the temperatures can exceed 400°C, but more plausibly on the distal, off-axis, portions of hydrothermal systems where the temperature is lower (Shock 1990, 1992a, b). Greater activities of the precursor molecules, CH2O and HCN, can co-exist in equilibrium with CO, CO2, H2, and N2 as measured in modern hydrothermal systems at cooler temperatures. Activities of HCN corresponding to concentrations in the millimolar range have been calculated to exist at the lower end of the temperature spectrum considered in this study regardless of whether it was generated from CO or CO2. However, the concentrations of CO, CO2, H2, and N2 from a modern hydrothermal system may not be representative of past geologic conditions. If the concentrations of these building block molecules would be higher, as hypothesized for the early-Earth atmosphere (Kasting 1993), then higher activities of CH2O and HCN could then coexist in equilibrium with CO, CO2, H2, and N2. It follows that the synthetic potential for the nucleobases, ribose and deoxyribose would also be greater than what is reported in the present study. Therefore, we have explored the range of CH2O and HCN activities on the equilibrium activities of nucleobase, ribose, and deoxyribose at what can be considered one particular hydrothermal flank condition (150°C and 500 bars). Under these conditions, activities of all of the biomolecules considered here are in equilibrium with the precursor molecules at concentrations comparable to those in modern living organisms (Voet et al. 1999).
Complementing other studies that have shown that various biological and organic molecules such as amino acids (Amend and Shock 2000), carboxylic acids, alcohols, and ketones (Shock and Schulte 1998) can be synthesized in hydrothermal systems, the results of this study support the hypothesis that hydrothermal systems could have served as efficient anabolic reactors for building the molecules that are essential to living organisms. The thermodynamic calculations shown here also provide constraints on the temperature, pressure and bulk composition necessary for the synthesis of these fundamental biomolecules in hydrothermal systems. Our results provide a useful theoretical framework for the design of experiments aimed at synthesizing nucleobases and sugars from inorganic precursors.
References
Amend JP, Plyasunov AV (2001) Carbohydrates in thermophile metabolism: calculation of the standard molal thermodynamic properties of aqueous pentoses and hexoses at elevated temperatures and pressures. Geochim Cosmochim Acta 65(21):3901–3917
Amend JP, Shock EL (1998) Energetics of amino acid synthesis in hydrothermal ecosystems. Science 281(5383):1659–1662
Amend JP, Shock EL (2000) Thermodynamics of amino acid synthesis in hydrothermal ecosystems on the early Earth. In: Goodfriend G (ed) Perspectives in amino acid and protein geochemistry. Plenum, New York, pp 23–40
Bock GR, Goode JA (1996) Evolution of hydrothermal ecosystems on Earth (and Mars?). Wiley, New York
Butlerov A (1861) Formation synthétique d’une substance sucrée. Comptes Rendus Acad Sci 53:145–147
Charlou JL, Donval JP, Fouquet Y, Jean-Baptiste P, Holm NG (2002) Geochemistry of high H2 and CH4 vent fluids issuing from ultramafic rocks at the Rainbow hydrothermal field (36°14¢N, MAR). Chem Geol 191:345–359
Delano JW (2001) Redox history of the Earth’s interior since ×3900 Ma: implications for prebiotic molecules. Orig Life Evol Biosph 31:311–341
Ferris JP (2005) Catalysis and prebiotic synthesis. Rev Mineral Geochem 59:187–210
Ferris JP, Sanchez RA, Orgel LE (1968) Studies in prebiotic synthesis. 3. Synthesis of pyrimidines from cyanoacetylene and cyanate. J Mol Biol 33:693–704
Ferris JP, Joshi PC, Edelson EH, Lawless JG (1978) HCN: a plausible source of purines, pyrimidines and amino acids on the primitive Earth. J Mol Evol 11:293–311
Foustoukos DI, Seyfried WE (2004) Hydrocarbons in hydrothermal vent fluids: the role of chromium-bearing catalysts. Science 304:1002–1005
Franiatte M, Richard L, Elie M, Nguyen-Trung C, Perfetti E, LaRowe DE (2008) Hydrothermal stability of adenine under controlled fugacities of N2, CO2, and H2. Orig Life Evol Biosph 38(2):139–148 doi:10.1007/s11084-008-9126-5
Hayatsu R, Studier MH, Oda A, Fuse K, Anders E (1968) Origin of organic matter in early solar system—II. Nitrogen compounds. Geochim Cosmochim Acta 32:175–190
Hazen RM, Boctor N, Brandes JA, Cody GD, Hemley RJ, Sharma A, Yoder HS (2002) High pressure and the origin of life. J Phys Condens Matter 14:11489–11494
Hennet RJC, Holm NG, Engel MH (1992) Abiotic synthesis of amino acids under hydrothermal conditions and the origin of life—a perpetual phenomenon. Naturwissenschaften 79:361–365
Hill A, Orgel LE (2002) Synthesis of adenine from HCN tetramer and ammonium formate. Orig Life Evol Biosph 32:99–102
Holm NG (1992) Why are hydrothermal systems proposed as plausible environments for the origin of life. Orig Life Evol Biosph 22:5–14
Holm NG, Andersson EM (1995) Abiotic synthesis of organic compounds under the conditions of submarine hydrothermal systems: a perspective. Planet Space Sci 43:153–159
Holm NG, Andersson E (2005) Hydrothermal simulation experiments as a tool for studies of the origin of life on Earth and other terrestrial planets: a review. Astrobiology 5(4):444–460
Holm NG, Dumont M, Ivarsson M, Konn C (2006) Alkaline fluid circulation in ultramafic rocks and formation of nucleotide constituents: a hypothesis. Geochem Trans 7:1–7
Johnson JW, Oelkers EH, Helgeson HC (1992) SUPCRT92—a software package for calculating the standard molal thermodynamic properties of minerals, gases, aqueous species, and reactions from 1 bar to 5000 bar and 0°C to 1000°C. Comput Geosci 18(7):899–947
Kasting JF (1993) Earth’s early atmosphere. Science 259:920–926
LaRowe DE, Helgeson HC (2006) Biomolecules in hydrothermal systems: calculation of the standard molal thermodynamic properties of nucleic-acid bases, nucleosides, and nucleotides at elevated temperatures and pressures. Geochim Cosmochim Acta 70:4680–4724
LaRowe DE, Helgeson HC (2007) Quantifying the energetics of metabolic reactions in diverse biogeochemical systems: electron flow and ATP synthesis. Geobiology 5:153–168
Marshall WL (1994) Hydrothermal synthesis of amino acids. Geochem Cosmochim Acta 9:2099–2106
McCollom TM (2003) Formation of meteorite hydrocarbons from thermal decomposition of siderite (FeCO3). Geochim Cosmochim Acta 67:311–317
McCollom TM, Amend JP (2005) A thermodynamic assessment of energy requirements for biomass synthesis by chemolithoautotrophic micro-organisms in oxic and anoxic environments. Geobiology 3:135–144
McCollom TM, Seewald JS (2001) A reassessment of the potential for reduction of dissolved CO2 to hydrocarbons during serpentinization of olivine. Geochim Cosmochim Acta 65:3769–3778
McCollom TM, Seewald JS (2003a) Experimental constraints on the hydrothermal reactivity or organic acids and acid anions: I. Acetic acid, acetate, and valeric acid. Geochim Cosmochim Acta 67:3645–3664
McCollom TM, Seewald JS (2003b) Experimental constraints on the hydrothermal reactivity or organic acids and acid anions: I. Formic acid and formate. Geochim Cosmochim Acta 67:3625–3644
McCollom TM, Seewald JS (2007) Abiotic synthesis of organic compounds in deep-sea hydrothermal environments. Chem Rev 107:382–401
McCollom TM, Simoneit BRT (1999) Abiotic formation of hydrocarbons and oxygenated compounds during the thermal decomposition of iron oxalate. Orig Life Evol Biosph 29:167–186
McCollom TM, Ritter G, Simoneit BRT (1999) Lipid synthesis under hydrothermal conditions by Fischer–Tropsch-type reactions. Orig Life Evol Biosph 29:153–166
Miller SL (1957) The mechanism of synthesis of amino acids by electric discharges. Biochem Biophys Acta 23:480–489
Miyakawa S, Murasawa K, Kobayashi K, Sawaoka AB (2000) Abiotic synthesis of guanine with high-temperature plasma. Orig Life Evol Biosph 30:557–566
Müller D, Pitsch S, Kittaka A, Wagner E, Wintner CE, Eschenmoser A, Ohlofjgewidmet G (1990) Chemie von a-aminonitrilen. Aldomerisierung von glycolaldehyd-phosphat zu racemischen hexose-2,4,6-triphosphaten und (in gegenwart von formaldehyd) racemischen pentose-2,4-diphosphaten: rac-allose-2,4,6-triphosphat und rac-ribose-2,4-diphosphat sind die reaktionshauptprodukte. Helv Chim Acta 73:1410–1468
Nisbet EG, Fowler CMR (1996) The hydrothermal imprint on life: did heat-shock proteins, metalloproteins and photosynthesis begin around hydrothermal vents? In: MacLeod CJ, Tyler PA, Walker CL (eds) Tectonic, magmatic, hydrothermal and biological segmentation of Mid-Ocean ridges. Geological Society, London, pp 239–251
Orgel LE (2004) Prebiotic chemistry and origin of the RNA world. Crit Rev Biochem Mol Biol 39:99–123
Oró J (1960) Synthesis of adenine from hydrogen cyanide. Biochem Biophys Res Commun 2:407–412
Oró J, Kimball AP (1961) Synthesis of purines under possible primitive Earth conditions, I. Adenine from hydrogen cyanide. Arch Biochem Biophys 94:217–227
Ricardo A, Carrigan MA, Olcott AN, Benner SA (2004) Borate minerals stabilize ribose. Science 303:196
Rushdi AI, Simoneit BRT (2001) Lipid formation by aqueous Fischer–Tropsch-type synthesis over a temperature range of 100 to 400 degrees C. Orig Life Evol Biosph 31:103–118
Rushdi AI, Simoneit BRT (2004) Condensation reactions and formation of amides, esters, and nitriles under hydrothermal conditions. Astrobiology 4:211–224
Rushdi AI, Simoneit BRT (2005) Abiotic synthesis of organic compounds from carbon disulfide under hydrothermal conditions. Astrobiology 5:749–769
Saladino R, Ciambecchini U, Crestini C, Costanzo G, Negri R, DiMauro E (2003) One-pot-TiO2-catalyzed synthesis of nucleic bases and acyclonucleosides from formamide: implications for the origin of life. ChemBioChem 4:514–521
Saladino R, Crestini C, Ciambecchini U, Ciciriello F, Costanzo G, DiMauro E (2004) Synthesis and degradation of nucleobases and nucleic acids by formamide in the presence of montmorillonites. ChemBioChem 5:1558–1566
Saladino R, Crestini C, Costanzo G, DiMauro E (2005a) On the prebiotic synthesis of nucleobases, nucleotides, oligonucleotides, pre-RNA and pre-DNA molecules. Top Curr Chem 259:29–68
Saladino R, Crestini C, Neri V, Brucato JR, Colangeli L, Ciciriello F, DiMauro E, Costanzo G (2005b) Synthesis and degradation of nucleic acid components by formamide and cosmic dust analogues. ChemBioChem 6:1368–1374
Schulte MD, Rogers KL (2004) Thiols in hydrothermal solution: standard partial molal properties and their role in the organic geochemistry of hydrothermal environments. Geochim Cosmochim Acta 68(5):1087–1097
Schulte MD, Shock EL (1993) Aldehydes in hydrothermal solution—standard partial molal thermodynamic properties and relative stabilities at high temperatures and pressures. Geochim Cosmochim Acta 57(16):3835–3846
Schulte MD, Shock EL (1995) Thermodynamics of Strecker synthesis in hydrothermal systems. Orig Life Evol Biosph 25:161–173
Shapiro R (1988) Prebiotic ribose synthesis: a critical analysis. Orig Life Evol Biosph 18:71–85
Shapiro R (1995) The prebiotic role of adenine: a critical analysis. Orig Life Evol Biosph 25:83–98
Shock EL (1990) Geochemical constraints on the origin of organic compounds in hydrothermal systems. Orig Life Evol Biosph 20(3–4):331–367
Shock EL (1992a) Chemical environments of submarine hydrothermal systems. Orig Life Evol Biosph 22(1–4):67–107
Shock EL (1992b) Hydrothermal organic synthesis experiments. Orig Life Evol Biosph 22(1–4):135–146
Shock EL (1996) Hydrothermal systems as environments for the emergence of life. In: Bock GR, Goode JA (eds) Evolution of hydrothermal ecosystems on Earth (and mars?). Wiley, New York, pp 40–60
Shock EL, Helgeson HC (1990) Calculation of the thermodynamic and transport properties of aqueous species at high pressures and temperatures—standard partial molal properties of organic species. Geochim Cosmochim Acta 54(4):915–945
Shock EL, McKinnon WB (1993) Hydrothermal processing of cometary volatiles—application to Triton. Icarus 106:464–477
Shock EL, Schulte MD (1990) Summary and implications of reported amino acid concentrations in the Murchison meteorite. Geochim Cosmochim Acta 54:3159–3173
Shock EL, Schulte MD (1993) Aldehydes in hydrothermal solution—standard partial molal thermodynamic properties and relative stabilities at high temperatures and pressures. Geochim Cosmochim Acta 57:3835–3846
Shock EL, Schulte MD (1995) Thermodynamics of Strecker synthesis in hydrothermal systems. Orig Life Evol Biosph 25:161–173
Shock EL, Schulte MD (1998) Organic synthesis during fluid mixing in hydrothermal systems. J Geophys Res 103(E12):28513–28527
Shock EL, Amend JP, Zolotov MY (2000) The early Earth vs. the origin of life. In: Canup RM, Righter K (eds) Origin of the Earth and Moon. University of Arizona Press, Tucson, AZ, pp 527–543
Shvarov YV, Bastrakov EN (1999) HCh: a software package for geochemical equilibrium modelling. User’s guide: Australian Geological Survey Organisation, record 1999/25
Simoneit BRT (2004) Prebiotic organic synthesis under hydrothermal conditions: an overview. Adv Space Res 33:88–94
Tanger JC, Helgeson HC (1988) Calculation of the thermodynamic and transport properties of aqueous species at high pressures and temperatures—revised equations of state for the standard partial molal properties of ions and electrolytes. Am J Sci 288(1):19–98
Voet D, Voet JG, Pratt CW (1999) Fundamentals of biochemistry. Wiley, New York
Von Damm KL (1995) Temporal and compositional diversity in sea-floor hydrothermal fluids. Rev Geophys 33(Suppl. S):1297–1305
Wakamatsu H, Yamada Y, Saito T, Kumashiro I, Takenishi T (1966) Synthesis of adenine by oligomerization of hydrogen cyanide. J Org Chem 31:2035–2036
Williams LB, Canfield B, Voglesonger KM, Holloway JR (2005) Organic molecules formed in a “primordial womb”. Geology 33:913–916
Woese C (1998) The universal ancestor. Proc Natl Acad Sci USA 95:6854–6859
Woese CR, Kandler O, Wheelis ML (1990) Towards a natural system of organisms—proposal for the domains Archaea, Bacteria, and Eucarya. Proc Nat Acad Sci USA 87(12):4576–4579
Zolotov MY, Shock EL (1999) Abiotic synthesis of polycyclic aromatic hydrocarbons on Mars. J Geophys Res 104:14033–14049
Zubay G (1998) Studies on the lead-catalyzed synthesis of aldopentoses. Orig Life Evol Bios 28:13–26
Zubay G, Mui T (2001) Prebiotic synthesis of nucleotides. Orig Life Evol Bios 31:87–102
Acknowledgments
This work is supported by the Netherlands Organization for Scientific Research (NWO) grant number 815.01.008.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
LaRowe, D.E., Regnier, P. Thermodynamic Potential for the Abiotic Synthesis of Adenine, Cytosine, Guanine, Thymine, Uracil, Ribose, and Deoxyribose in Hydrothermal Systems. Orig Life Evol Biosph 38, 383–397 (2008). https://doi.org/10.1007/s11084-008-9137-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11084-008-9137-2