Abstract
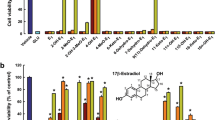
In the present work, potential protective effects of quercitrin (a phytoestrogen) on Aβ-induced neurotoxicity in cultured rat hippocampal neurons were investigated in comparison with 17β-estradiol. Cell viability, oxidative status, and antioxidative potentials were used as comparative parameters. Co-exposure of cultured neurons to Aβ25–35 with either quercitrin or 17β-estradiol (50–100 μM) for 72 h attenuated Aβ25–35-induced neurotoxicity and lipid peroxidation, but not Aβ25–35-induced ROS accumulation. However, only 17β-estradiol counteracted a reduction in glutathione content and only quercitrin counteracted a reduction in glutathione peroxidase activity. Both compounds displayed no effects on superoxide dismutase activity. A specific estrogen receptor antagonist, ICI 182780, did not abolish neuroprotective effects of quercitrin and 17β-estradiol. These findings suggested that quercitrin and 17β-estradiol attenuated Aβ25–35-induced neurotoxicity in a comparable manner. Underlying neuroprotective mechanisms of both compounds were probably not related to estrogen receptor-mediated genomic mechanisms but might involve with their antioxidant and free radical scavenging properties.








Similar content being viewed by others
References
Selkoe DJ, Schenk D (2003) Alzheimer’s disease: molecular understanding predicts amyloid-based therapeutics. Annu Rev Pharmacol Toxicol 43:545–584
Kar S, Slowikowski SPM, Westaway D et al (2004) Interactions between β-amyloid and central cholinergic neurons: implications for Alzheimer’s disease. J Psychiatr Neurosci 29:427–441
Khan AA, Mao XO, Banwait S et al (2007) Neuroglobin attenuates β-amyloid neurotoxicity in vitro and transgenic Alzheimer phenotype in vivo. Proc Natl Acad Sci USA 104:19114–19119
Frozza RL, Horn AP, Hoppe JB et al (2009) A comparative study of β-amyloid peptides Aβ1–42 and Aβ25–35 toxicity in organotypic hippocampal slice cultures. Neurochem Res 34:295–303
Lesné S, Koh MT, Kotilinek L et al (2006) A specific amyloid-β protein assembly in the brain impairs memory. Nature 440:352–357
Jang J-H, Surh Y-J (2005) β-Amyloid-induced apoptosis is associated with cyclooxygenase-2 up-regulation via the mitogen activated protein kinase-NFκB signaling pathway. Free Radic Biol Med 38:1604–1613
Abe K, Saito H (2000) Amyloid β neurotoxicity not mediated the mitogen-activated protein kinase cascade in cultured rat hippocampal and cortical neurons. Neurosci Lett 292:1–4
Kosuge Y, Koen Y, Ishige K et al (2003) S-allyl-l-cysteine selectively protects cultured rat hippocampal neurons from amyloid β-protein- and tunicamycin-induced neuronal death. Neuroscience 122:885–895
Korol TY, Korol SV, Kostyuk EP et al (2008) β-Amyloid-induced changes in calcium homeostasis in cultured hippocampal neurons of the rat. Neurophysiology 40:9–12
Xiao XQ, Wang R, Han YF et al (2000) Protective effects of huperzine A on β-amyloid25–35 induced oxidative injury in rat pheochromocytoma cells. Neurosci Lett 286:155–158
Kim H, Bang OY, Jung MW et al (2001) Neuroprotective effects of estrogen against beta-amyloid toxicity are mediated by estrogen receptors in cultured neuronal cells. Neurosci Lett 302:58–62
Keelan J, Allen NJ, Antcliffe D et al (2001) Quantitative imaging of glutathione in hippocampal neurons and glia in culture using monochlorobimane. J Neurosci Res 66:873–884
Jayakumar R, Murali J, Koteeswari D et al (2004) Cytotoxic and membrane perturbation effects of a novel amyloid forming model peptide poly (leucine-glutamic acid). J Biochem 136:457–462
Dong YL, Zuo PP, Li Q et al (2007) Protective effects of phytoestrogen α-zearalanol on beta amyloid25–35 induced oxidative damage in cultured rat hippocampal neurons. Endocrinology 32:206–211
Dhitavat S, Orti D, Rogers E et al (2005) Folate, vitamin E, and acetyl-l-carnitine provide synergistic protection against oxidative stress resulting from exposure of human neuroblastoma cells to amyloid-beta. Brain Res 1061:114–117
Nilsen J, Chen S, Irwin RW et al (2006) Estrogen protects neuronal cells from amyloid beta-induced apoptosis via regulation of mitochondrial proteins and function. BMC Neurosci 7:74–87
Roth A, Schaffner W, Hertel C (1999) Phytoestrogen kaempferol (3, 4′, 5, 7-tetrahydroxyflavone) protects PC12 and T47D cells from beta-amyloid-induced toxicity. J Neurosci Res 57:399–404
Wang CN, Chi CW, Lin YL et al (2001) The neuroprotective effects of phytoestrogens on amyloid β protein-induced toxicity are mediated by abrogating the activation of caspase cascade in rat cortical neurons. J Biol Chem 276:5287–5295
Zeng H, Chen Q, Zhao B (2004) Genistein ameliorates β-amyloid peptide (25–35)-induced hippocampal neuronal apoptosis. Free Radic Biol Med 36:180–188
Bang OY, Hong HS, Kim DH et al (2004) Neuroprotective effect of genistein against beta amyloid-induced neurotoxicity. Neurobiol Dis 16:21–28
Wagner C, Fachinetto R, Dalla Corte CL et al (2006) Quercitrin, a glycoside form of quercetin, prevents lipid peroxidation in vitro. Brain Res 1107:192–198
Kraus B, Wolff H, Heilmann J et al (2007) Influence of Hypericum perforatum extract and its single compounds on amyloid-β mediated toxicity in microglial cells. Life Sci 81:884–894
Hollman PC, de Vries JH, Van Leeuwen SD et al (1995) Absorption of dietary quercetin glycosides and quercetin in healthy ileostomy volunteers. Am J Clin Nutr 62:1276–1282
Morand C, Manach C, Crespy V et al (2000) Quercetin 3-O-beta-glucoside is better absorbed than other quercetin forms and is not present in rat plasma. Free Radic Res 33:667–676
Unchern S, Nagata K, Saito H (1997) Selective cytotoxicity of piperine on cultured rat hippocampal neurons in comparison with cultured astrocytes: the possible involvement of lipid peroxidation. Biol Pharm Bull 20:958–961
Pike C, Overman MJ, Cotman CW (1995) Amino terminal detection enhanced aggregation of beta amyloid peptide in vitro. J Biol Chem 270:23895–23898
Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 65:55–63
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–358
Bastianetto S, Zheng WH, Quirion R (2000) The Ginkgo biloba extract (EGb 761) protects hippocampal neurons against cell death induced by beta-amyloid. Eur J Neurosci 12:1882–1890
Tietze F (1969) Enzymic method for quantitative determination of nanogram amounts of total and oxidized glutathione: applications to mammalian blood and other tissues. Anal Biochem 27:502–522
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein dye binding. Anal Biochem 72:248–254
Jiang X, Mu D, Manabat C et al (2004) Differential vulnerability of immature murine neurons to oxygen-glucose deprivation. Exp Neurol 190:224–232
Shirai K, Mizui T, Suzuki Y et al (2006) Differential effects of x-irradiation on immature and mature hippocampal neurons in vitro. Neurosci Lett 399:57–60
Behl C, Skutella T, Lezoualch F et al (1997) Neuroprotection against oxidative stress by estrogens: structure–activity relationship. Mol Pharmacol 51:535–541
Yagyu K, Kitagawa K, Irie T et al (2001) Amyloid proteins inhibit Cl–ATPase activity in cultured rat hippocampal neurons. J Neurochem 78:569–576
Fitzpatrick JL, Mize AL, Wade CB et al (2002) Estrogen-mediated neuroprotection against beta-amyloid toxicity requires expression of estrogen receptor alpha or beta and activation of MAPK pathway. J Neurochem 82:674–682
Quintanilla RA, Muñoz FJ, Metcalfe MJ et al (2005) Trolox and 17β-estradiol protect against amyloid β-peptide neurotoxicity by a mechanism that involves modulation of the Wnt signaling pathway. J Biol Chem 280:11615–11625
Canevari L, Abramov AY, Duchen MR (2004) Toxicity of amyloid β peptide: tales of calcium, mitochondria, and oxidative stress. Neurochem Res 29:637–650
Benzi G, Moretti A (1995) Are reactive oxygen species involved in Alzheimer’s disease? Neurobiol Aging 16:661–674
Lovell MA, Ehmann WD, Butler SM et al (1995) Elevated thiobarbituric acid-reactive substances and antioxidant enzyme activity in the brain in Alzheimer’s disease. Neurology 45:1594–1601
Marcus DL, Thomas C, Rodriguez C et al (1998) Increased peroxidation and reduced antioxidant enzyme activity in Alzheimer’s disease. Exp Neurol 150:40–44
Butterfield DA, Lauderback CM (2002) Lipid peroxidation and protein oxidation in Alzheimer’s disease brain: potential causes and consequences involving amyloid β-peptide-associated free radical oxidative stress. Free Radic Bio Med 32:1050–1060
Bae YH, Hwang JY, Kim YH et al (2000) Antioxidative neuroprotection by estrogens in mouse cortical cultures. J Korean Med Sci 15:327–336
Cecchi C, Latorraca S, Sorbi S et al (1999) Glutathione level is altered in lymphoblasts from patients with familial Alzheimer’s disease. Neurosci Lett 275:152–154
Abramov AY, Canevari L, Duchen MR (2003) Changes in intracellular calcium and glutathione in astrocytes as the primary mechanism of amyloid neurotoxicity. J Neurosci 23:5088–5095
Reiter RJ (1995) Oxidative processes and antioxidative defense mechanisms in the aging brain. FASEB J 9:526–533
McEwen B (2002) Estrogen actions throughout the brain. Recent Prog Horm Res 57:357–384
Dubal DB, Shughrue PJ, Wilson ME et al (1999) Estradiol modulates bcl-2 in cerebral ischemia: a potential role for estrogen receptors. J Neurosci 19:6385–6393
Sawada H, Ibi M, Kihara T et al (2000) Mechanisms of antiapoptotic effects of estrogens in nigral dopaminergic neurons. FASEB J 14:1202–1214
Wilson ME, Dubal DB, Wise PM (2000) Estradiol protects against injury-induced cell death in cortical explant cultures: a role for estrogen receptors. Brain Res 873:235–242
Lee SY, Andoh T, Murphy DL et al (2003) 17β-Estradiol activates ICI 182, 780-sensitive estrogen receptors and cyclic GMP-dependent thioredoxin expression for neuroprotection. FASEB J 17:947–948
Howard SA, Brooke SM, Sapolsky RM (2001) Mechanisms of estrogenic protection against gp 120-induced neurotoxicity. Exp Neurol 168:385–391
Wang X, Dykens JA, Perez E et al (2006) Neuroprotective effects of 17β-estradiol and nonfeminizing estrogens against H2O2 toxicity in human neuroblastoma SK-N-SH cells. Mol Pharmacol 70:395–404
Behl C, Widmann M, Trapp T et al (1995) 17-beta estradiol protects neurons from oxidative stress-induced cell death in vitro. Biochem Biophys Res Comm 216:473–482
Xu C, Bailly-Maitre B, Reed JC (2005) Endoplasmic reticulum stress: cell life and death decisions. J Clin Invest 115:2656–2664
Yoshida H (2007) ER stress and diseases. FEBS J 274:630–658
Imai T, Kosuge Y, Ishige K et al (2007) Amyloid β-protein potentiates tunicamycin-induced neuronal death in organotypic hippocampal slice cultures. Neuroscience 147:639–651
Natsume Y, Ito S, Satsu H et al (2009) Protective effect of quercetin on ER stress caused by calcium dynamics dysregulation in intestinal epithelial cells. Toxicology 258:164–175
Acknowledgments
This study was supported partly by the Graduate Research Funds from the Graduate School, Chulalongkorn University.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rattanajarasroj, S., Unchern, S. Comparable Attenuation of Aβ25–35-Induced Neurotoxicity by Quercitrin and 17β-Estradiol in Cultured Rat Hippocampal Neurons. Neurochem Res 35, 1196–1205 (2010). https://doi.org/10.1007/s11064-010-0175-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-010-0175-6