Abstract
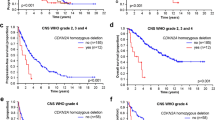
Glutamine, glutamate, asparagine, and aspartate are involved in an enzyme-network that controls nitrogen metabolism. Branched-chain-amino-acid aminotransferase-1 (BCAT1) promotes proliferation of gliomas with wild-type IDH1 and is closely connected to the network. We hypothesized that metabolism of asparagine, glutamine, and branched-chain-amino-acids is associated with progression of malignant gliomas. Gene expression for asparagine synthetase (ASNS), glutaminase (GLS), and BCAT1 were analyzed in 164 gliomas from 156 patients [33-anaplastic gliomas (AG) and 131-glioblastomas (GBM), 64 of which were recurrent GBMs]. ASNS and GLS were twofold higher in GBMs versus AGs. BCAT1 was also higher in GBMs. ASNS expression was twofold higher in recurrent versus new GBMs. Five patients had serial samples: 4-showed higher ASNS and 3-higher GLS at recurrence. We analyzed grade and treatment in 4 groups: (1) low ASNS, GLS, and BCAT1 (n = 96); (2) low ASNS and GLS, but high BCAT1 (n = 26); (3) high ASNS or GLS, but low BCAT1 (n = 25); and (4) high ASNS or GLS and high BCAT1 (n = 17). Ninety-one % of patients (29/32) with grade-III lesions were in group 1. In contrast, 95 % of patients (62/65) in groups 2–4 had GBMs. Treatment was similar in 4 groups (radiotherapy-80 %; temozolomide-30 %; other chemotherapy-50 %). High expression of ASNS, GLS, and BCAT1 were each associated with poor survival in the entire group. The combination of lower ASNS, GLS, and BCAT1 levels correlated with better survival for newly diagnosed GBMs (66 patients; P = 0.0039). Only tumors with lower enzymes showed improved outcome with temozolomide. IDH1WT gliomas had higher expression of these genes. Manipulation of amino acid metabolism in malignant gliomas may be further studied for therapeutics development.



Similar content being viewed by others
Abbreviations
- AA:
-
Anaplastic astrocytoma
- AG:
-
Anaplastic glioma
- AMG:
-
Anaplastic mixed glioma
- AO:
-
Anaplastic oligodendroglioma
- ASN:
-
Asparagine
- ASNS:
-
Asparagine synthetase
- BCAA:
-
Branched chain amino acids
- BCAT:
-
Branched chain amino-acid aminotransferase
- GBM:
-
Glioblastoma multiforme
- GLN:
-
Glutamine
- GLS:
-
Glutaminase
- IDH:
-
Isocitrate dehydrogenase
- IDH1WT :
-
Isocitrate dehydrogenase 1, wild type
References
Wolf A, Agnihotri S, Guha A (2010) Targeting metabolic remodeling in glioblastoma multiforme. Oncotarget 1(7):567
la Fougère C, Suchorska B, Bartenstein P, Kreth F-W, Tonn J-C (2011) Molecular imaging of gliomas with PET: opportunities and limitations. Neuro-Oncology 13:806–819. doi:10.1093/neuonc/nor054
Langen K-J, Tatsch K, Grosu A-L, Jacobs AH, Weckesser M, Sabri O (2008) Diagnostics of cerebral gliomas with radiolabeled amino acids. Dtsch Arztebl Int 105:55–61. doi:10.3238/arztebl.2008.0055
Galldiks N, Langen K (2014) Applications of PET imaging of neurological tumors with radiolabeled amino acids. Q J Nucl Med Mol Imaging 59(1):70–82
Dranoff G, Elion GB, Friedman HS, Bigner DD (1985) Combination chemotherapy in vitro exploiting glutamine metabolism of human glioma and medulloblastoma. Cancer Res 45:4082–4086
Panosyan EH, Wang Y, Xia P, Lee W-NP, Pak Y, Laks DR, Lin HJ, Moore TB, Cloughesy TF, Kornblum HI, Lasky JL (2014) Asparagine depletion potentiates the cytotoxic effect of chemotherapy against brain tumors. Mol Cancer Res 12:694–702. doi:10.1158/1541-7786.mcr-13-0576
Seltzer MJ, Bennett BD, Joshi AD, Gao P, Thomas AG, Ferraris DV, Tsukamoto T, Rojas CJ, Slusher BS, Rabinowitz JD, Dang CV, Riggins GJ (2010) Inhibition of glutaminase preferentially slows growth of glioma cells with mutant IDH1. Cancer Res 70:8981–8987. doi:10.1158/0008-5472.can-10-1666
Taylor SA, Crowley J, Pollock TW, Eyre HJ, Jaeckle C, Hynes HE, Stephens RL (1991) Objective antitumor activity of acivicin in patients with recurrent CNS malignancies: a Southwest Oncology Group trial. J Clin Oncol 9:1476–1479
Chang SM, Kuhn JG, Robins HI, Schold SC, Spence AM, Berger MS, Mehta MP, Bozik ME, Pollack I, Schiff D, Gilbert M, Rankin C, Prados MD (1999) Phase II study of phenylacetate in patients with recurrent malignant glioma: a North American brain tumor consortium report. J Clin Oncol 17:984
Tonjes M, Barbus S, Park YJ, Wang W, Schlotter M, Lindroth AM, Pleier SV, Bai AHC, Karra D, Piro RM, Felsberg J, Addington A, Lemke D, Weibrecht I, Hovestadt V, Rolli CG, Campos B, Turcan S, Sturm D, Witt H, Chan TA, Herold-Mende C, Kemkemer R, Konig R, Schmidt K, Hull W-E, Pfister SM, Jugold M, Hutson SM, Plass C, Okun JG, Reifenberger G, Lichter P, Radlwimmer B (2013) BCAT1 promotes cell proliferation through amino acid catabolism in gliomas carrying wild-type IDH1. Nat Med 19:901–908. doi:10.1038/nm.3217.html#supplementary-information.
Balasubramanian MN, Butterworth EA, Kilberg MS (2013) Asparagine synthetase: regulation by cell stress and involvement in tumor biology 304(8):E789–E799
Wise DR, Thompson CB (2010) Glutamine addiction: a new therapeutic target in cancer. Trends Biochem Sci 35:427–433. doi:10.1016/j.tibs.2010.05.003
Agnihotri S, Aldape KD, Zadeh G (2014) Isocitrate dehydrogenase status and molecular subclasses of glioma and glioblastoma. Neurosurg Focus 37:E13. doi:10.3171/2014.9.focus14505
Reitman ZJ, Jin G, Karoly ED, Spasojevic I, Yang J, Kinzler KW, He Y, Bigner DD, Vogelstein B, Yan H (2011) Profiling the effects of isocitrate dehydrogenase 1 and 2 mutations on the cellular metabolome. Proc Natl Acad Sci 108:3270–3275. doi:10.1073/pnas.1019393108
Venneti S, Thompson CB (2013) Metabolic modulation of epigenetics in gliomas. Brain Pathol 23:217–221. doi:10.1111/bpa.12022
Bender DA (2012) Amino acids synthesized from glutamate: glutamine, proline, ornithine, citrulline and arginine. Amino acid metabolism. Wiley, New York, pp 157–223
Zhang J, Fan J, Venneti S, Cross Justin R, Takagi T, Bhinder B, Djaballah H, Kanai M, Cheng Emily H, Judkins Alexander R, Pawel B, Baggs J, Cherry S, Rabinowitz Joshua D, Thompson Craig B (2014) Asparagine plays a critical role in regulating cellular adaptation to glutamine depletion. Mol Cell 56:205–218. doi:10.1016/j.molcel.2014.08.018
Lee Y, Scheck A, Cloughesy T, Lai A, Dong J, Farooqi H, Liau L, Horvath S, Mischel P, Nelson S (2008) Gene expression analysis of glioblastomas identifies the major molecular basis for the prognostic benefit of younger age. BMC Med Genom 1:52
Mareninov S, De Jesus J, Sanchez D, Kay A, Wilson R, Babic I, Chen W, Telesca D, Lou J, Mirsadraei L, Gardner T, Khanlou N, Vinters H, Shafa B, Lai A, Liau L, Mischel P, Cloughesy T, Yong W (2013) Lyophilized brain tumor specimens can be used for histologic, nucleic acid, and protein analyses after 1 year of room temperature storage. J Neurooncol 113:365–373. doi:10.1007/s11060-013-1135-1
Chou AP, Chowdhury R, Li S, Chen W, Kim AJ, Piccioni DE, Selfridge JM, Mody RR, Chang S, Lalezari S, Lin J, Sanchez DE, Wilson RW, Garrett MC, Harry B, Mottahedeh J, Nghiemphu PL, Kornblum HI, Mischel PS, Prins RM, Yong WH, Cloughesy T, Nelson SF, Liau LM, Lai A (2012) Identification of retinol binding protein 1 promoter hypermethylation in isocitrate dehydrogenase 1 and 2 mutant gliomas. J Natl Cancer Inst 104:1458–1469. doi:10.1093/jnci/djs357
Weller M, Cloughesy T, Perry JR, Wick W (2013) Standards of care for treatment of recurrent glioblastoma—are we there yet? Neuro-Oncology 15:4–27. doi:10.1093/neuonc/nos273
Squatrito M, Holland EC (2011) DNA damage response and growth factor signaling pathways in gliomagenesis and therapeutic resistance. Cancer Res 71:5945–5949. doi:10.1158/0008-5472.can-11-1245
Khasraw M, Ameratunga MS, Grant R, Wheeler H, Pavlakis N (2014) Antiangiogenic therapy for high-grade glioma. Cochrane Database Syst Rev 9:CD008218. doi:10.1002/14651858.CD008218.pub3
Patel M, Kim J, Ruzevick J, Li G, Lim M (2014) The future of glioblastoma therapy: synergism of standard of care and immunotherapy. Cancers 6:1953–1985
Marie SKN, Shinjo SMO (2011) Metabolism and brain cancer. Clinics 66:33–43
Weinberg SE, Chandel NS (2015) Targeting mitochondria metabolism for cancer therapy. Nat Chem Biol 11:9–15. doi:10.1038/nchembio.1712
Avramis VI, Panosyan EH (2005) Pharmacokinetic/pharmacodynamic relationships of asparaginase formulations: the past, the present and recommendations for the future. Clin Pharmacokinet 44:367–393
Stathias V, Pastori C, Griffin TZ, Komotar R, Clarke J, Zhang M, Ayad NG (2014) Identifying glioblastoma gene networks based on hypergeometric test analysis. PLoS ONE 9:e115842. doi:10.1371/journal.pone.0115842
Hart MG, Garside R, Rogers G, Stein K, Grant R (2013) Temozolomide for high grade glioma. Cochrane Database Syst Rev 4:CD007415. doi:10.1002/14651858.CD007415.pub2
Wu S-H, Bi J-F, Cloughesy T, Cavenee WK, Mischel PS (2014) Emerging function of mTORC2 as a core regulator in glioblastoma: metabolic reprogramming and drug resistance. Cancer Biol Med 11:255–263. doi:10.7497/j.issn.2095-3941.2014.04.004
Gao Q, Lei T, Ye F (2013) Therapeutic targeting of EGFR-activated metabolic pathways in glioblastoma. Expert Opin Investig Drugs 22:1023–1040. doi:10.1517/13543784.2013.806484
R2: Genomics Analysis and Visualization Platform (http://r2.amc.nl). http://hgserver1.amc.nl/cgi-bin/r2/main.cgi
Gross MI, Demo SD, Dennison JB, Chen L, Chernov-Rogan T, Goyal B, Janes JR, Laidig GJ, Lewis ER, Li J, MacKinnon AL, Parlati F, Rodriguez MLM, Shwonek PJ, Sjogren EB, Stanton TF, Wang T, Yang J, Zhao F, Bennett MK (2014) Antitumor activity of the glutaminase inhibitor CB-839 in triple-negative breast cancer. Mol Cancer Ther 13:890–901. doi:10.1158/1535-7163.mct-13-0870
Funding
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Eduard H. Panosyan: Consultation and compensation from: Baxter, Octapharma, CSL Behring, Jazz Pharmaceuticals. Joseph L. Lasky: Consultation and compensation from: Baxter, Octapharma, CSL Behring. Henry J. Lin: None. Albert Lai: Consultancy with Genentech/Roche, research funding from Takeda/Millennium and Genentech/Roche. Yang Hai: None. Xiuqing Guo: None. Michael Quinn: Contracts with the following academic medical centers: Ronald Reagan UCLA Medical Center, Keck School of Medicine of University of Southern California and Kaiser Permanente. No industry sponsored contracts. Stanley F. Nelson: None. Timothy F. Cloughesy: Consultant with and compensation from: Roche, Genentech, Upshire Smith, VBL, Abbvie, Nektar, Novocure, Tocagen, Notable labs, Novartis, Celgene, Lpath, Proximagen, Amgen, Newgen. Expert testimony: Roche. Phioanh L. Nghiemphu: None.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Panosyan, E.H., Lasky, J.L., Lin, H.J. et al. Clinical aggressiveness of malignant gliomas is linked to augmented metabolism of amino acids. J Neurooncol 128, 57–66 (2016). https://doi.org/10.1007/s11060-016-2073-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-016-2073-5