Abstract
Phylogenetic analysis based on the deduced amino acid sequence of phenylalanine ammonia-lyase gene (SlPAL5) cDNA from tomato (Solanum lycopersicum L.) revealed high sequence similarity to PAL genes in Nicotiana tabacum (92%), Ipomoea nil (87%), Manihot esculenta (84%), and Catharanthus roseus (84%). The SlPAL5 gene exists as multiple copies in the tomato plant, and its transcription was strongly expressed in old leaves and flowers. From 5 days post-anthesis to the onset of ripening, SlPAL5 expression decreased gradually but was maintained at a comparatively high level; SlPAL5 transcript expression was very low at the mature-green stage. SlPAL5 expression was significantly induced in response to NaCl, mannitol, and cold treatment; SlPAL5 expression decreased gradually after treatment with abscisic acid and H2O2; SlPAL5 transcript decreased after exposure to methyl viologen for 3 h and increased after 6 h and maintained a stable expression level until 24 h, suggesting that the SlPAL5 gene may function in the response to abiotic stress.
Similar content being viewed by others
Introduction
Plants exhibit a variety of responses to mechanical stress that enables them to tolerate and resist diverse abiotic environmental stresses such as severe temperature changes, drought, and salinity [1]. To survive these stresses, plants have developed a diverse and complex set of defense mechanisms [2]. Understanding the function of stress-inducible genes should help to clarify mechanisms of stress tolerance. Some genes are induced by abiotic stress, which can protect cells via the generation of important metabolic and cellular protection proteins and can regulate some genes that are involved in the transduction of stress response signals [3].
The phenylpropanoid pathway constitutes a major secondary metabolic pathway in plants, leading to the synthesis of a variety of compounds, e.g., flavonoids and lignins [4]. Plants produce phenylpropanoid compounds in response to infections by fungi or bacteria and are considered responsible for plant defense mechanisms [5]. Moreover, phenylpropanoid compounds play a variety of roles in plant reproduction and development and are induced in plants by various environmental stresses [6, 7]. Induction of phenylpropanoid synthesis under stress conditions is caused by increased transcription of genes encoding the corresponding biosynthetic enzymes.
Phenylalanine ammonia-lyase (PAL) is a key enzyme in phenylpropanoid metabolism and catalyzes the conversion of phenylalanine to trans-cinnamic acid, the first step in the biosynthesis of phenylpropanoid. PAL genes constitute a small gene family in plants and are found in bean, parsley, rice, potato, and Arabidopsis [8]. PAL activity seems to be extraordinarily sensitive to the physiological state of the plant. Changes in PAL activity can occur during growth or may follow traumatic or pathological events or the action of light [9]. Red light, acting via phytochrome, stimulates PAL activity in the cotyledons and hypocotyls of tomato seedlings, and exposure to UV-B has been shown to stimulate PAL activity in rice, maize, and turnip [10, 11]. PAL activity can be induced by various stresses such as chilling, wounding, ozone, pathogen invasion [12, 13], the plant hormone ethylene, and plant signal molecules, including jasmonic acid, salicylic acid, and MeJA [14, 15]. Anthocyanin and PAL activity in strawberry plants is increased when treated with abscisic acid (ABA) [16].
As the plant phenylpropanoid plays a wide range of biological roles, there has been great interest in understanding their biosynthetic regulation. We are interested in the regulation of PAL expression since the vast majority of phenylpropanoid compounds are synthesized through PAL. PAL expression is regulated by combinations of various developmental and environmental stimuli; hence, it is important to understand how PAL expression is regulated in a whole organism throughout development. Furthermore, the environmental stresses that induce PAL transcripts have not been fully elucidated.
Here we investigated the expression of SlPAL5 during tomato development and under various environmental stresses. The environmental stresses used in this study included 200 mM NaCl, cold (4°C), 200 mM mannitol, 100 μM ABA, 10 mM H2O2, and 50 μM methyl viologen (MV). Our findings suggest that PAL plays an important role in multiple signaling pathways activated by abiotic stress.
Materials and methods
Plant materials and treatments
Tomato (Solanum lycopersicum L.) seeds were cultured in Murashige and Skoog (MS) medium (including 3% sucrose, 0.8% agar, pH 5.8). The germinated plants were transferred to pots and kept in a growth chamber at 24°C for 4 weeks. For cold treatment, the leaves were placed in distilled water and kept in a 4°C cold chamber under dim light for 24 h; for other environmental stresses treatments, they were incubated in 200 mM NaCl, 200 mM mannitol, 10 mM H2O2, or 50 μM MV separately for various durations. The ABA stock solution was prepared by dissolving ABA in small aliquots of 1 N NaOH. The ABA was applied to detached leaves through the petiole after dilution.
Multiple amino acid sequence alignment
SlPAL5 cDNA has been isolated from tomato and a BLASTP search was conducted against proteins in the NR database for matching proteins [17]. Multiple sequence alignments of tomato PAL and other PAL proteins from other plants were generated using http://us.expasy.org/tools. The accession numbers are: BAA22948 (Nicotiana tabacum), AAG49585 (Ipomoea nil), AAK62030 (Manihot esculenta), and BAA95629 (Catharanthus roseus). Phylogenic tree of SlPAL5 homologs in N. tabacum, I. nil, M. esculenta, and C. roseus was drawn using the DNAMAN program.
RNA isolation and obtain of the full-length cDNA of SlPAL5
Forward (5′-ATGGATTTGTGCAAGAAATC-3′) and reverse (5′-TTAGCAGATTGGAAGAGG-3′) primers were designed to amplify the complete, full-length PAL gene from tomato cDNA by RT-PCR. Total RNA was isolated from young tomato leaves using TRI-reagent® according to the manufacturer’s instructions (MRC, USA). From the DNase-treated total RNA (1 μg), first-strand cDNA was synthesized using the AccuPower® PCR PreMix (Bioneer, South Korea) containing oligo(dT) primers, and Moloney murine leukemia virus reverse transcriptase (M-MLV RT, Invitrogen, USA).
The PCR reaction was carried out as follows: initial 5 min of denaturation at 94°C; followed by 30 cycles at 94°C for 1 min, 55°C for 1 min, 72°C for 1 min; and a final 7 min at 72°C. Twelve microliters of the reaction products were separated on 1% agarose gels and visualized after staining with ethidium bromide. All experiments were performed in triplicate.
DNA isolation and Southern blotting analysis
Genomic DNA was isolated from mature tomato leaves. Genomic DNA samples (20 μg) were digested to completion with EcoRI, HindIII, and XbaI. Digested genomic DNA was separated by electrophoresis on a 1% agarose gel, denatured, and blotted onto a nylon membrane (Amersham Pharmacia, Uppsala, Sweden). Southern blotting was conducted, and membranes were hybridized with the full-length of SlPAL5 cDNA probe labeled with [α-32P] dCTP. Hybridization was performed overnight at 65°C in 5% dextran sulfate, 0.25 M disodium phosphate (pH 7.2), 7% (w/v) SDS, and 1 mM EDTA. After hybridization, the blot was washed twice with 2 × SSC and 0.1% SDS for 10 min each at room temperature and twice with 0.1 × SSC and 0.1% SDS for 5 min each at 65°C. The blots were then dried and placed on X-ray film at −80°C for 1 week for development.
Northern blotting analysis
Total RNA concentrations and purity were determined by spectrophotometry and by staining ribosomal RNA with ethidium bromide, respectively. Equal quantities of total RNA were loaded on 1% agarose gels containing 7.4% formaldehyde. After electrophoresis and visualization under UV light, the RNA was transferred to nylon membranes (Hybond N+, Amersham) and hybridization was performed using a SlPAL5 cDNA probe labeled with [α-32P] dCTP. After hybridization, the blot was washed twice with 2 × SSC and 0.1% SDS for 10 min each at room temperature and twice with 0.1 × SSC and 0.1% SDS for 5 min each at 65°C. The blots were then dried and placed on X-ray film at −80°C for 1 week for development.
Results and discussion
Sequence analysis of SlPAL5
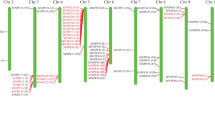
A full-length 2,115 bp PAL cDNA was obtained (GenBank accession no. M83314). There are five different classes of the PAL genomic sequence in tomato [18]. Our target gene showed the highest identity (93%) to tomato PAL5 (P26600), suggesting that it belongs to the PAL5 group. An 11-base pair exonic regulatory element (nucleotides 67 to 77) exists in the gene sequence and plays a role in the transcriptional pathogen response. Phylogenetic analysis based on the deduced amino acid sequence of SlPAL5 cDNA revealed high sequence similarity to other PAL genes (Fig. 1a). It was 92% identical to N. tabacum PAL (BAA22948), 87% identical to I. nil (AAG49585), 87% identical to M. esculenta (AAK62030), and 84% to C. roseus PAL (BAA95629). Among tomato PAL and PAL proteins from other plants, the first 20 amino acids of the N-terminal domains are very different, while the other downstream residues are highly conserved in all PAL proteins. The evolutionary relationship among these five PAL proteins is shown in Fig. 1b.
Multiple alignment of SlPAL5 with other PAL proteins. (a) Comparison of the derived amino acid sequences of SlPAL5 with other PAL proteins in Nicotiana tabacum (BAA22948), Ipomoea nil (AAG49585), Manihot esculenta (AAK62030), and Catharanthus roseus (BAA95629). Residues shaded in black are identical between the different proteins. (b) Phylogenic tree of SlPAL5 homologs in N. tabacum, I. nil, M. esculenta, and C. roseus. The dendrogram was drawn using the DNAMAN program. The bootstrap values are in percent
Southern blotting analysis of SlPAL5
To assess the copy number of the PAL gene in tomato, Southern blotting analysis was performed on tomato genomic DNA digested with EcoRI, HindIII, and XbaI using a SlPAL5 full-length cDNA probe. Hybridization of the genomic DNA blot with a probe encompassing the full-length cDNA of SlPAL5 resulted in multiple bands (Fig. 2a). This finding indicates the presence of the SlPAL5 gene family in the genome of tomato. As mentioned above, our target gene showed the highest identity with tomato PAL5, suggesting that it belongs to the PAL5 group. The double bands that existed in genomic DNA digested with EcoRI and HindIII further support this conclusion.
Gel blotting analysis of SlPAL5. (a) Southern blotting analysis: genomic DNA was digested with EcoRI, HindIII, and XbaI, loaded on an agarose gel, and hybridized with the 32P-labeled probe corresponding to the full-length SlPAL5 cDNA. (b) Tissue RNA expression of the SlPAL5 gene: SlPAL5 RNA (20 μg) levels were monitored in different plant organs (OL, mature tomato leaves; YL, young tomato leaves; F, flowers; R, roots; S, stems)
Northern blotting analysis of SlPAL5 expression in various tissues in different stages
Northern blotting analysis with RNA isolated from various tissues (e.g., mature and young leaves, flowers, roots, and stems) from non-stressed tomato plants was performed using a SlPAL5 probe (Fig. 2b). The SlPAL5 transcript was strongly expressed in mature tomato leaves and flowers. A comparatively strong signal was observed in young leaves and roots, but no signal was detected in stems. High steady-state levels of the PAL transcript have been found in the roots and flowers of tobacco plants [5]. However, in that study, the PAL transcript level was very low in mature leaves, which is opposite of our findings in tomato. PAL transcripts accumulate differently in various organs of the mature tomato plant, and different type of PAL gene expression profiles differ in the same organ [19], implying that there expression is controlled by distinct regulatory.
Expression pattern of SlPAL5 during tomato development
During development, fruit growth was followed by measuring tomato diameter from anthesis to the mature-green stage (Fig. 3a). The expression of SlPAL5 transcripts at various stages of tomato development was analyzed by Northern blotting (Fig. 3b). The SlPAL5 gene was highly expressed in flowers, but was expressed at very low levels in the mature-green stage. From 5 days post-anthesis to the onset of ripening, SlPAL5 expression decreased gradually but was nevertheless maintained at a comparatively high level. Our results were consistent with the findings in strawberry. The PAL protein activity during anthesis has been suggested to be involved in the synthesis of flavonoids and phenolics during early fruit development, whereas in near ripe fruits, is correlated with the anthocyanin accumulation that is the hallmark of ripe fruits [19, 20]. Our results indicate distinct patterns of developmental regulation of the PAL gene in tomato flowers and fruits.
Expression pattern of SlPAL5 during tomato development. (a) Development of tomato: the growth curve was established by measuring tomato diameters daily at the following developmental stages: anthesis, 5, 10, 15, and 20 DPA (days post-anthesis), mature-green stages. Data are the average of five distinct measurements. (b) Northern blotting analysis of the SlPAL5 genes in tomato. Total RNA (20 μg) was isolated loaded on an agarose gel, and hybridized with the 32P-labeled probe corresponding to the full-length SlPAL5 cDNA
Expression of SlPAL5 mRNA in response to various abiotic stresses
To determine whether the SlPAL5 gene affects the responses of plants to abiotic stress, the expression of this gene under various abiotic stresses, including 200 mM NaCl, 200 mM mannitol, cold (4°C), and 100 μM ABA treatments, was investigated by Northern blotting (Fig. 4). Distilled water treatment was used as a control condition for abiotic stress (Fig. 4a). In response to NaCl and mannitol treatment (Fig. 4b, d), the SlPAL5 transcript increased significantly after 1 h of treatment and began to decline gradually from then on. In cold-treated tomato leaves, the SlPAL5 transcripts were induced within 3 h and reached a maximum by 6 h (Fig. 4c).
Expression of the SlPAL5 gene in tomato leaf tissues that were exposed to various abiotic stresses. (a) D.W. treatment was used as control. (b) NaCl (200 mM), (c) cold treatment at 4°C, (d) mannitol (200 mM), (e) ABA (100 μM) were used as the abiotic stresses. Total RNA (20 μg) from leaf samples at various time points after treatment was loaded into each lane, and SlPAL5 cDNA was used as a probe. To ensure equal loading of RNA, a duplicate gel was stained with ethidium bromide as an RNA loading control
Abscisic acid is an important plant hormone involved in adaptive responses of plants to various environmental conditions. The SlPAL5 transcript level decreased after ABA treatment (Fig. 4e). Most of the genes that respond to drought, salt, and cold stress are induced by exogenous application of ABA [21]. However, several genes that are induced by water stress are not responsive to exogenous ABA treatment. These findings suggest the existence of both ABA-independent and -dependent signal transduction cascades between the initial signal of drought or cold stress and the expression of specific genes [22]. The SlPAL5 transcripts increased significantly after treatment with various abiotic stresses but not by ABA, which suggests that at least two separate regulatory systems control gene expression during drought and cold stress. These observations provide evidence that the SlPAL5 gene is related to NaCl, cold, and mannitol stresses and may therefore belong to an ABA-independent regulation system. The mechanisms underlying the activation of the abiotic stress response by SlPAL5 remain to be elucidated in detail.
Expression of SlPAL5 mRNA in response to various oxidative stresses
The expression pattern of the SlPAL5 gene in tomato plants may be related to responses to oxidative stress, as suggested by results from Northern blotting analysis (Fig. 5). Treatment with 10 mM H2O2 caused the SlPAL5 transcript level to decrease after 3 h. A similar decrease was observed for the SlPAL5 transcript after exposure to MV for 3 h; however, the transcripts increased after 6 h and maintained a stable expression level until 24 h.
All organisms produce reactive oxygen species (ROS) as byproducts of normal metabolic processes, including superoxide anion radicals (O2−), hydroxyl radicals (•OH), hydrogen peroxide (H2O2), and singlet oxygen (O2 1) [23]. ROS, especially H2O2, have roles in the responses of plants to both abiotic and biotic stresses and can be both toxic and beneficial [24]. H2O2 possesses direct antimicrobial activity, is involved in signal transduction pathways triggered during stress responses, and interacts with other signaling molecules such as reactive nitrogen species [25]. MV reduces O2 and generates superoxide anion in the chloroplast [26]. Plant injury caused by environmental stresses may be related to ROS-initiated oxidative damage at the cellular level [27, 28]. Plants possess a wide range of enzymatic and non-enzymatic mechanisms to scavenge ROS, including specific antioxidant enzymes and low-molecular weight antioxidants such as phenolic compounds, glutathione, and vitamin C [29]. However, the accumulation of phenolic compounds during oxidative stress is not consistent with the increase in PAL transcripts, suggesting that other pathways and processes contribute to the accumulation of phenolic compounds during oxidative stress.
In the present study, we investigated the expression of the SlPAL5 gene during abiotic and oxidative stress by Northern blotting. SlPAL5 was significantly induced by NaCl, cold, and mannitol stress, but not by the plant hormone ABA. These observations suggest that the SlPAL5 gene is related to responses to abiotic stress and may belong to an ABA-independent regulation system. The mechanisms underlying the activation of the abiotic response by SlPAL5 remain to be fully elucidated.
References
Yi SY, Kim JH, Joung YH, Lee S, Kim WT, Yu SH et al (2004) The pepper transcription factor CaPF1 confers pathogen and freezing tolerance in Arabidopsis. Plant Physiol 136:2862–2874. doi:10.1104/pp.104.042903
Dangl JL, Jones JDG (2001) Plant pathogens and integrated defense responses to infection. Nature 411:826–833. doi:10.1038/35081161
Umezawa T, Fujita M, Fujita Y, Yamaguchi-Shinozaki K, Shinozaki K (2006) Engineering drought tolerance in plants: discovering and tailoring genes to unlock the future. Curr Opin Biotechnol 17:113–122
Weisshaar B, Jenkins GI (1998) Phenylpropanoid biosynthesis and its regulation. Curr Opin Plant Biol 1:251–257. doi:10.1016/S1369-5266(98)80113-1
Fukasawa-Akada T, Kung SD, Watson JC (1996) Phenylalanine ammonia-lyase gene structure Expression and evolution in Nicotiana. Plant Mol Biol 30:711–722. doi:10.1007/BF00019006
Stewart AJ, Chapman W, Jenkins GI, Graham T, Martin T, Crozier A (2001) The effect of nitrogen and phosphorus deficiency on flavonol accumulation in plant tissue. Plant Cell Environ 24:1189–1197. doi:10.1046/j.1365-3040.2001.00768.x
Dixon RA, Paiva NL (1995) Stress-induced phenylpropanoid metabolism. Plant Cell 7:1085–1097
Wanner LA, Li G, Ware D, Somssich IE, Davis KR (1995) The phenylalanine ammonia-lyase gene family in Arabidospis thaliana. Plant Mol Biol 27:327–338. doi:10.1007/BF00020187
Brődenfeldt R, Mohr H (1988) Time courses for phytochrome-induced enzyme levels in phenylpropanoid metabolism (phenylalanine ammonia-lyase, naringenin-chalcone synthase) compared with time courses for phytochrome-mediated end-product accumulation (anthocyanin, quercetin). Planta 41(176):383–390. doi:10.1007/BF00395419
Reddy VS, Goud KV, Sharma R, Reddy AR (1994) UV-B responsive anthocyanin production in a rice cultivar is associated with a specific phase of phenylalanine ammonia lyase biosynthesis. Plant Physiol 105:1059–1066
Singh A, Selvi MT, Sharma R (1999) Sunlight-induced anthocyanin pigmentation in maize vegetative tissues. J Exp Bot 50:1619–1625. doi:10.1093/jexbot/50.339.1619
Lafuente MT, Zacarias L, Martinez-Telez MA, Sanchez-Ballesta MT, Granell A (2003) Phenylalanine ammonia-lyase and ethylene in relation to chilling injury as affected by fruit age in citrus. Postharvest Biol Technol 29:308–317. doi:10.1016/S0925-5214(03)00047-4
Campos-Vargas R, Nonogaki H, Suslow T, Saltveit ME (2005) Heat shock treatments delay the increase in wound induced phenylalanine ammonia-ammonia-lyase activity by altering its expression, not its induction in Romaine lettuce (Lactuca sativa) tissue. Physiol Plant 132:82–91. doi:10.1111/j.1399-3054.2005.00446.x
Campos-Vargas R, Saltveit ME (2002) Involvement of putative chemical wound signals in the induction of phenolic metabolism in wounded lettuce. Physiol Plant 114:73–84. doi:10.1034/j.1399-3054.2002.1140111.x
Chen JY, Wen PF, Kong WF, Pan QH, Zhan JC, Li JM et al (2006) Effect of salicylic acid on phenylpropanoids and phenylalanine ammonia-lyase in harvested grape berries. Postharvest Biol Technol 40:64–72. doi:10.1016/j.postharvbio.2005.12.017
Jiang YM, Joyce DC (2003) ABA effects on ethylene production, PAL activity, anthocyanin and phenolic contents of strawberry fruit. Plant Growth Regul 39:171–174. doi:10.1023/A:1022539901044
Lee S, Kim SY, Chung E, Joung YH, Pai HS, Hur CG et al (2004) EST and microarray analyses of pathogen responsive genes in hot pepper (Capsicum annuum L.) non-host resistance against soybean pustule pathogen (Xanthomonas axonopodis pv. glycines). Funct Integr Genomics 4:196–205. doi:10.1007/s10142-003-0099-1
Lee SW, Robb J, Nazar RN (1992) Truncated phenylalanine ammonia-lyase expression in tomato (Lycopersicon esculentum). J Biol Chem 267:11824–11830
Kumar A, Ellis BE (2001) The phenylalanine ammonia-lyase gene family in raspberry Structure, expression, and evolution. Plant Physiol 127:230–239. doi:10.1104/pp.127.1.230
Chen JY, He LH, Jiang YM, Wang Y, Joyce DC, Ji ZL et al (2008) Role of phenylalanine ammonia-lyase in heat pretreatment-induced chilling tolerance in banana fruit. Physiol Plant 132:318–328. doi:10.1111/j.1399-3054.2007.01013.x
Shinozaki K, Yamaguchi-Shinozaki K (1996) Molecular responses to drought and cold stress. Curr Opin Biotechnol 7:161–167. doi:10.1016/S0958-1669(96)80007-3
Bray EA (1997) Plant responses to water deficit. Trends Plant Sci 2:48–54. doi:10.1016/S1360-1385(97)82562-9
Foyer CH, Descourvieres P, Kunert KJ (1994) Protection against oxygen radicals: an important defense mechanism studied in transgenic plants. Plant Cell Environ 17:507–523. doi:10.1111/j.1365-3040.1994.tb00146.x
Dat J, Vandenabeels S, Vranova E, Van Montagu M, Inze D, Van Breusegem F (2000) Dual action of the active oxygen species during plant stress responses. Cell Mol Life Sci 57:779–795. doi:10.1007/s000180050041
Willekens H, Inze D, Van Montagu M, Van Camp W (1995) Catalases in plants. Mol Breed 1:207–228. doi:10.1007/BF02277422
Babbs CF, Pham JA, Coolbaugh RC (1989) Lethal hydroxyl radical production in paraquat-treated plants. Plant Physiol 90:1267–1270
Kuzniak E (2002) Transgenic plants: an insight into oxidative stress tolerance mechanisms. Acta Physiol Plant 24:97–113. doi:10.1007/s11738-002-0027-3
Robinson JM, Bunce JA (2000) Influence of drought-induced water stress on soybean and spinach leaf ascorbate-dehydroascorbate level and redox status. Int J Plant Sci 161:271–279. doi:10.1086/314257
Wang FZ, Wang QB, Kwon SY, Kwak SS, Su WA (2005) Enhanced drought tolerance of transgenic rice plants expressing a pre management superoxide dismutase. J Plant Physiol 162:465–472. doi:10.1016/j.jplph.2004.09.009
Acknowledgments
This work was supported by the Basic Research Promotion Fund (KRF-2007-313-C00688) in South Korea.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Guo, J., Wang, MH. Characterization of the phenylalanine ammonia-lyase gene (SlPAL5) from tomato (Solanum lycopersicum L.). Mol Biol Rep 36, 1579–1585 (2009). https://doi.org/10.1007/s11033-008-9354-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-008-9354-9