Abstract
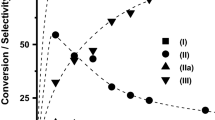
The synthesis of 1,5 benzodiazepine using natural and modified Argentinean bentonite (pillared layered clay and porous clay heterostructure) as catalysts through a condensation reaction between o-phenylenediamine (o-PDA) and excess of acetone as reactive and solvent at room temperature is reported. The catalysts were found to be highly active and selective, affording a high yield of the corresponding benzodiazepine. The effects of the modification of the natural bentonite and reaction conditions, such as temperature, time and amount of catalyst were investigated. The catalysts were also successfully employed for the preparation of other derivatives of 1,5-benzodiazepine using substituted o-PDAs and ketones. In all cases, the reactions are highly selective and are completed within 1–3 h. The catalyst showed excellent activity in all cases, showing 86–90% isolated yields of the corresponding derivatives of 1,5-benzodiazepine. The easy work-up procedure and the recyclable catalyst make this methodology attractive for large-scale operations.










Similar content being viewed by others
References
Z. Ding, H.Y. Zhu, P.F. Greenfield, J. Colloid Interface Sci. 209, 193–199 (1999)
C. Ooka, H. Yoshida, K. Suzuki, T. Hattori, Appl. Catal. A Gen. 260, 47–53 (2004)
H.Y. Zhu, Z. Ding, J.C. Barry, J. Phys. Chem. B 106, 11420–11429 (2002)
H. Mao, B. Li, X. Li, L. Yue, Z. Liu, M. Wei, Ind. Eng. Chem. Res. 49 583–591 (2010), and references cited herein
P. Yuan, H. He, F. Bergaya, D. Wu, Q. Zhou, J. Zhu, Micropor. Mesopor. Mater. 88, 8–15 (2006)
R. Zhu, T. Wang, F. Ge, W. Chen, Z. You, J. Colloid Interface Sci. 335, 77–83 (2009)
R. Zhu, L. Zhu, J. Colloid Surf. Sci. 322, 27–32 (2008)
T. An, J. Chen, G. Li, X. Ding, G. Sheng, J. Fu, B. Mai, K. O’Shea, Catal. Today 139, 69–76 (2008)
A. Elmchaouri, R. Mahboub, Colloids Surf. A 259, 135–141 (2005)
J.Q. Jiang, C. Cooper, S. Ouki, Chemosphere 47, 711–716 (2002)
J. Ma, L. Zhu, J. Hazard. Mater. 136, 982–988 (2006)
K.R. Srinivasan, S.H. Fogler, Clays Clay Miner. 38, 277–286 (1990)
R.S. Varma, K. Pitchumani, K.P. Naicker, Green Chem. 95 (1999) and references cited herein
J.K. Landquist, in Comprehensive heterocyclic Chem., eds. by A.R. Katritzky, C.W. Rees (Pergamon, Oxford, 1984), Vol. 1, pp 166–170
H. Schutz, Benzodiazepines (Springer, Heidelberg, 1982)
A.M. El-Sayed, A. Khodairy, H. Salah, H. Abdel-Ghany, Phosphorus Sulfur Silicon Relat. Elem. 182, 711–722 (2007)
K. Nabih, A. Baouid, A. Hasnaoui, A. Kenz, Synth. Commun. 34, 3565–3572 (2004)
G.K. Nagaraja, V.P. Vaidya, K.S. Rai, K.M. Mahadevan, Phosphorus Sulfur Silicon Relat. Elem. 181, 2297–2806 (2006)
K.V.V. Reddy, P.S. Rao, D. Ashok, Synth. Commun. 30, 1825–1836 (2000)
C.W. Kuo, C.C. Wang, V. Kavala, C.F. Yao, Molecules 13 2313–2325 (2008) , and references cited herein
J.S. Yadav, B.V.S. Reddy, G. Satheesh, G. Srinivasulu, A.C. Kunwar, ARKIVOC III, 221–227 (2005)
X.Q. Pan, J.P. Zou, Z. Huang, W. Zhang, Tetrahedron Lett. 49 5302–5308 (2008), and references cited herein
J.A.L. Herbert, H. Suschitzky, J. Chem, Soc. Perkin Trans. 1, 2657–2661 (1974)
M. Curini, F. Epifano, M.C. Marcotullio, O. Rosati, Tetrahedron Lett. (42) 18 3193–3195 (2001) Corrigendum to Tetrahedron Lett. 42 (2001) 3193–3195 Tetrahedron Lett. (42) 27(2001) 4593
S.K. De, R. Gibbs, Tetrahedron Lett. 46, 1811–1813 (2005)
J. Wu, F. Xu, Z. Zhou, Q. Shen, Synth. Commun. 36, 457–464 (2006)
W. Zhong, Y. Zhang, X. Chen, Tetrahedron Lett. 42, 73–75 (2001)
G. Sabitha, K.B. Reddy, M.N. Reddy, J.S. Yadav, Adv. Synth. Catal. 346, 1447–1453 (2004)
R. Varala, R. Enugala, S. Nuvula, S.R. Adapa, Synlett 7, 1009–1014 (2006)
M.M. Heravi, V. Zadsirjan, F.K. Behbahani, H.A. Oskooie, J. Mol. Catal. A Chem. 259, 201–204 (2006)
D.I. Jung, T.W. Choi, Y.Y. Kim, I.S. Kim, Y.M. Park, Y.G. Lee, D.H. Jung, Synth. Commun. 29, 1941–1951 (1999)
M. Benjelloum, P. Cool, P. Van Der Voort, E.F. Vansant, Phys. Chem. Chem. Phys. 4 2818–2823 (2002), and references cited herein
A. Gil, L.M. Gandia, M.A. Vicente, Catal. Rev. Sci. Eng. 42 145–212 (2000), and references cited herein
J.T. Kloprogge, L.V. Duong, R.L. Frost, Environ. Geol. 47, 967–981 (2005)
F. Kooli, P. Cheng Hian, Q. Weirong, S.F. Alshahateet, C. Martin, V. Rives, Clay Science 12 Sup. 2 295–300 (2006), and references cited herein
E.P. Barrett, L.G. Joyner, P.P. Halenda, J. Am. Chem. Soc. 73, 373–375 (1951)
B.C. Lippens, J.H. de Boer, J. Catal. 4, 319–323 (1965)
W.D. Harkins, G. Jura, J. Am. Chem. Soc. 66, 1366–1373 (1944)
L.J. Gurvitsch, Phys. Chem. Soc. Russ. 47, 805–827 (1915)
A. Samoson, E. Lippman, Phys. Rev. B, Condens. Matter 28 (1983) 6567
W. Yi, C. Cai, J. Fluor. Chem. 130, 1054–1058 (2009)
A. Galarneau, A.T. Barodawalla, J. Pinnavaia, Nature 374, 529–531 (1995)
R.S. Murray, J.P. Quirk, Langmuir 6, 122–124 (1990)
D. Massiot, F. Fayon, M. Capron, I. King, S. Le Calvet, B. Alonso, J.-O. Durand, B. Bujoli, Z. Gan, G. Hoatson, Magn. Reson. Chem. 40, 70 (2002)
R.S.Varma, K. Pitchumani, K.P. Naicker, Green Chem. 1 95 (1999), and references cited herein
Acknowledgments
We are grateful to Ing. Edgardo Soto, María E. Canafoglia and Diego Peña for their contributions in experimental measurements. Financial support from CONICET, CICPBA, MINCyT-ECOS SUD France and ANPCyT–PICT Projects (2494/06 and 1134/06) is gratefully acknowledged. C. I. Cabello is a member of the research staff of CICPBA, Argentina.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Carmen I. Cabello is a Member of CIC-PBA and Facultad de Ingeniería, UNLP.
Rights and permissions
About this article
Cite this article
Muñoz, M., Sathicq, G., Romanelli, G. et al. Porous modified bentonite as efficient and selective catalyst in the synthesis of 1,5-benzodiazepines. J Porous Mater 20, 65–73 (2013). https://doi.org/10.1007/s10934-012-9575-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10934-012-9575-0