Abstract
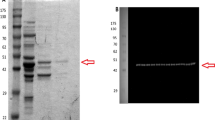
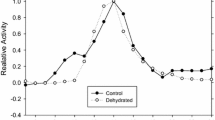
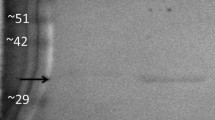
Glycerol-3-phosphate dehydrogenase (G3PDH; E.C.1.1.1.8) was purified from liver and skeletal muscle of black-tailed prairie dogs (Cynomys ludivicianus), a hibernating species. Native and subunit molecular masses of the dimeric enzyme were 77 and 40 kD, respectively, and both tissues contained a single isozyme with a pI of 6.4. Kinetic parameters of purified G3PDH from prairie dog liver and muscle were characterized at 22 and 5 °C and compared with rabbit muscle G3PDH. Substrate affinities for hibernator muscle G3PDH were stable (NAD) or increased significantly (Km G3P and DHAP decreased) at low temperature whereas Km NAD and DHAP of rabbit G3PDH increased. Prairie dog G3PDH showed greater conservation of Km G3P over a wide temperature range as well as greater thermal stability and resistance to chemical denaturation by guanidine hydrochloride than the rabbit enzyme. In addition, using the protein sequence of the hibernating thirteen-lined ground squirrel (Ictidomys tridecemlineatus) and bioinformatics tools, the deduced protein structure of G3PDH was compared between heterothermic and homeothermic mammals. Structural and functional characteristics of G3PDH from the hibernating species would support enzyme function over a wide range of core body temperatures over cycles of torpor and arousal.








Similar content being viewed by others
Abbreviations
- DHAP:
-
Dihydroxyacetone phosphate
- EDTA:
-
Ethylenediaminetetraacetic acid
- G3P:
-
Glycerol-3-phosphate
- G3PDH:
-
Glycerol-3-phosphate dehydrogenase
- HPLC:
-
High performance liquid chromatography
- SDS-PAGE:
-
Sodium dodecyl sulfate polyacrylamide gel electrophoresis
References
Arnold K, Bordoli L, Kopp J, Schwede T (2006) Bioinformatics 22:195–201
Arnórsdóttir J, Sigtryggsdóttir AR, Thorbjarnardóttir SH, Kristjánsson MM (2009) J Biochem 145:325–329
Benkert P, Biasini M, Schwede T (2011) Bioinformatics 27:343–350
Berrada W, Naya A, Ouafik L, Bourhim N (2000) Comp Biochem Physiol B 125:439–449
Berrada W, Naya A, Iddar A, Bourhim N (2002) Mol Cell Biochem 231:117–127
Bortz W, Paul P, Haff AC, Holmes WL (1972) Glycerol turnover and oxidation in man. J Clin Invest 51:1537–1546
Brooks SPJ (1992) BioTechniques 13:906–911
Dark J (2005) Annu Rev Nutr 25:469–497
Dark J, Ruby NF (1993) In: Carey C, Florant G, Wunder BA, Horowitz B (eds) Life in the cold. Westview Press, Boulder, pp 167–174
Eftink MR (1994) Biophys J 66:482–501
Galster WA, Morrison PR (1975) Am J Physiol 218:1228–1232
Geiser F (2004) Annu Rev Physiol 66:239–274
Green CJ, Brosnan JT, Fuller BJ, Lowry M, Stubbs M, Ross BD (1984) Comp Biochem Physiol B 79:167–171
Guex N, Peitsch MC (1997) Electrophoresis 18:2714–2723
Harlow HJ, Menkens GE (1986) Can J Zool 64:793–796
Lehmer EM, Savage LT, Antolin MF, Biggins DE (2006) Physiol Biochem Zool 79:454–467
McLoughlin DJ, MacQuarrie R (1978) Biochem Biophys Acta 527:204–211
Owen DE, Felig P, Morgan AP, Wahren J, Cahill GF (1969) Liver and kidney metabolism during prolonged starvation. J Clin Invest 48:574–583
Robinson-Rechavi M, Godzik A (2005) Structure 13:857–860
Sacchetta P, Aceto A, Bucciarelli T, Dragani B, Santarone S, Allocati N, Di-Ilio C (1993) Eur J Biochem 215:741–745
Schwede T, Kopp J, Guex N, Peitsch MC (2003) Nucleic Acids Res 31:3381–3385
Storey KB (1997) Comp Biochem Physiol A 118:1115–1124
Storey KB, Storey JM (2010) Adv Clin Chem 52:77–108
Vesterberg O (1971) In: Colowick SP, Kaplan NO (eds) Methods in enzymology. Academic Press, New York, pp 389–412
Wallace GM, Pfeiffer EW (1992) Comp Biochem Physiol A 101:853–855
White HB (1971) Arch Biochem Biophys 147:123–128
Acknowledgments
We thank Henry J. Harlow, University of Wyoming for providing the prairie dog tissue samples for this study. Thanks also to J. M. Storey for critical commentary on the manuscript. Supported by a research grant from the N.S.E.R.C. Canada to KBS. SNT held a NSERC PGSD scholarship.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
de la Roche, M., Tessier, S.N. & Storey, K.B. Structural and Functional Properties of Glycerol-3-Phosphate Dehydrogenase from a Mammalian Hibernator. Protein J 31, 109–119 (2012). https://doi.org/10.1007/s10930-011-9376-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10930-011-9376-3