Abstract
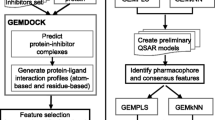
Inhibition of the enzyme acetylcholinesterase (AChE) has been shown to alleviate neurodegenerative diseases prompting several attempts to discover and optimize new AChE inhibitors. In this direction, we explored the pharmacophoric space of 85 AChE inhibitors to identify high quality pharmacophores. Subsequently, we implemented genetic algorithm-based quantitative structure–activity relationship (QSAR) modeling to select optimal combination of pharmacophoric models and 2D physicochemical descriptors capable of explaining bioactivity variation among training compounds (\( {\text{r}}^{ 2}_{ 6 8} = 0. 9 4 \), F-statistic = 125.8, \( {\text{r}}^{ 2}_{\text{LOO}} { = 0} . 9 2 \), \( {\text{r}}^{ 2}_{\text{PRESS}} \) against 17 external test inhibitors = 0.84). Two orthogonal pharmacophores emerged in the QSAR equation suggesting the existence of at least two binding modes accessible to ligands within AChE binding pocket. The successful pharmacophores were comparable with crystallographically resolved AChE binding pocket. We employed the pharmacophoric models and associated QSAR equation to screen the national cancer institute list of compounds. Twenty-four low micromolar AChE inhibitors were identified. The most potent gave IC50 value of 1.0 μM.





Similar content being viewed by others
References
Clark CM, Karlawish JH (2003) Ann Intern Med 138(5):400–410
Chen S, Zhang X-J, Li L, Le W-D (2007) Curr Neuropharmacol 5(2):127–134
Terry AV (2003) J Pharmacol Exp Ther 306(3):821–827
Bachurin SO (2003) Med Res Rev 23(1):48–88
Rollinger JM, Hornick A, Langer T, Stuppner H, Prast H (2004) J Med Chem 47(25):6248–6254
Perry EK, Kilford L, Lees AJ, Burn DJ, Perry RH (2003) Ann Neurol 54(2):235–238
Mukherjee PK, Satheeshkumar N, Venkatesh P, Venkatesh M (2011) Mini Rev Med Chem 11(3):247–262
Lu SH, Wu JW, Liu HL, Zhao JH, Liu KT, Chuang CK, Lin HY, Tsai WB, Ho Y (2011) J Biomed Sci 18:8–18
Whittaker VP (1990) Trends Pharmacol Sci 11(1):8–13
Purves D, George A, David F, William H, Anthony L, James M, Leonard W (2008) Neuroscience, 4th edn. Sinauer Associates Inc, Sunderland MA, pp 121–122
Quinn DM (1987) Chem Rev 87(5):955–979
Taylor P, Radic Z (1994) Annu Rev Pharmacol Toxicol 34:281–320
Sussman JL, Harel M, Frolow F, Oefner C, Goldman A, Toker L, Silman I (1991) Science 253(5022):872–879
Sussman JL, Harel M, Silman I (1993) Chem Biol Interact 87(1–3):187–197
Harel M, Schalk I, Ehret-Sabatier L, Bouet F, Goeldner M, Hirth C, Axelsen PH, Silman I, Sussman JL (1993) PNAS 90(19):9031–9035
Silman I, Harel M, Axelsen P, Raves M, Sussman JL (1994) Biochem Soc Trans 22(3):745–749
Harel M, Silman I, Quinn DM, Nair HK, Sussman JL (1996) J Am Chem Soc 118(10):2340–2346
Greenblatt HMKG, Lewis T, Silman I, Sussman JL (1999) FEBS Lett 463:321–326
Kryger G, Silman I, Sussman JL (1998) J Physiol 92(3–4):191–194
Inestrosa NC, Dinamarca MC, Alvarez A (2008) FEBS J 275(4):625–632
Rees T, Hammond PI, Soreq H, Younkin S, Brimijoin S (2003) Neurobiol Aging 24(6):777–787
Rees TM, Berson A, Sklan EH, Younkin L, Younkin S, Brimijoin S, Soreq H (2005) Curr Alzheimer Res 2(3):291–300
De Ferrari GV, Canales MA, Shin I, Weiner LM, Silman I, Inestrosa NC (2001) Biochemistry 40(35):10447–10457
National Pesticide Information Center-Diazinon Technical Fact Sheet (2012). http://npic.orst.edu/factsheets/diazinontech.pdf
Stoelting RK (1999) Anticholinesterase drugs and cholinergic agonists in pharmacology and physiology in anesthetic practice. Lippincott-Raven, Philadelphia
Taylor P, Hardman JG, Limbird LE, Molinoff PB., Ruddon RW, Gilman AG (1996) Autonomic pharmacology: cholinergic drugs the pharmacologial basis of therapeutics. The McGraw-Hill Companies, Columbus, Ohio
Taha MO, Bustanji Y, Al-Ghussein MAS, Mohammad M, Zalloum H, Al-Masri IM, Atallah N (2008) J Med Chem 51:2062–2077
CATALYST 4.11 Users’ Manual (2005) Accelrys Software Inc San Diego CA
Taha MO, Atallah N, Al-Bakri AG, Paradis-Bleau C, Zalloum H, Younis KS, Levesque RC (2008) Bioorg Med Chem 16(3):1218–1235
Taha MO, Bustanji Y, Al-Bakri AG, Yousef A-M, Zalloum WA, Al-Masri IM, Atallah N (2007) J Mol Graph Model 25(6):870–884
Al-masri IM, Mohammad MK, Taha MO (2008) ChemMedChem 3(11):1763–1779
Taha MO, Dahbiyeh LA, Bustanji Y, Zalloum H, Saleh S (2008) J Med Chem 51:6478–6494
Al-Nadaf AS, Taha MO (2010) Bioorg Med Chem 18:3088–3115
Abu-Hammad AM, Taha MO (2009) J Chem Inf Model 49:978–996
Abu Khalaf R, Abu Sheikha G, Bustanji Y, Taha MO (2010) Eur J Med Chem 45(4):1598–1617
Al-Sha’er MA, Taha MO (2010) Eur J Med Chem 45(9):4316–4330
Al-Sha’er MA, Taha MO (2010) J Chem Inf Model 50(9):1706–1723
Habash M, Taha MO (2011) Bioorg Med Chem 19(16):4746–4771
Shahin R, Alqtaishat S, Taha MO (2012) J Comput Aided Mol Des 26(2):249–266
Suaifan GARY, Shehadehh M, Al-Ijel H, Taha MO (2012) J Mol Graph Model 37:1–26
Shahin R, Taha MO (2012) Bioorg Med Chem 20(1):377–400
Taha MO, Qandil AM, Al-Haraznah T, Khalaf RA, Zalloum H, Al-Bakri AG (2011) Chem Biol Drug Des 78(3):391–407
Al-Nadaf AH, Taha MO (2011) J Mol Graph Model 29(6):843–864
Al-Najjar BO, Wahab HA, Tengku Muhammad TS, Shu-Chien AC, Ahmad Noruddin NA, Taha MO (2011) Eur J Med Chem 46(6):2513–2529
CERIUS2, QSAR Users’ Manual, version 4.10, Accelrys Inc., San Diego, CA (2005) 43–88, 221–235, 237–250
Discovery Studio 2.5.5 User Guide (2010) Accelrys Inc., San Diego
CS ChemDraw Ultra 6.0, Cambridge Soft Corp., USA
Fang L, Kraus B, Lehmann J, Heilmann J, Zhang Y, Decker M (2008) Bioorg Med Chem Lett 18(9):2905–2909
Fang L, Decker M, Roegler C, Lehmann J, Appenroth D, Fleck C, Kiehntopf M, Deufel T, Peng S, Zhang Y (2008) J Med Chem 51(4):713–716
Fang L, Zhang Y, Decker M, Appenroth D, Fleck C, Jumpertz S, Mohr K, Trankle C (2010) J Med Chem 53(5):2094–2103
Rook Y, Schmidtke K-U, Gaube F, Schepmann D, Wünsch B, Heilmann J, Lehmann J, Winckler T (2010) J Med Chem 53(9):3611–3617
Schott Y, Decker M, Rommelspacher H, Lehmann J (2006) Bioorg Med Chem Lett 16(22):5840–5843
Decker M, Krauth F, Lehmann J (2006) Bioorg Med Chem 14(6):1966–1977
Decker M, Kraus B, Heilmann J (2008) Bioorg Med Chem 16(8):4252–4261
Decker M (2006) J Med Chem 49(18):5411–5413
Sutter J, Guner O, Hoffmann R, Li H, Waldman M (2000). In: Guner OF (ed) Pharmacophore perception, development,and use in drug design. International University Line, La Jolla, pp 501–511
Kurogi Y, Güner OF (2001) Curr Med Chem 8(9):1035–1055
Poptodorov K, Luu T, Langer T, Hoffmann R (2006). In: Hoffmann RD (ed) Methods and principles in medicinal chemistry: pharmacophores and pharmacophores searches, vol 2. Wiley-VCH Weinheim, pp 17–47
Li H, Sutter J, Hoffmann R (2000) Pharmacophore perception development and use in drug design ed. International University Line, La Jolla, CA, pp 173–178
Bersuker I, Bahçeci S, Boggs J (2000) Pharmacophore perception development and use in drug design. International University Line, La Jolla, pp 457–473
Fischer R (1966) The principle of experimentation illustrated by a psycho-physical. ExpeHafner Publishing Co, 8th ed. Hafner Publishing,New York Chapter II
Krovat EM, Langer T (2003) J Med Chem 46(5):716–726
Triballeau N, Acher F, Brabet I, Pin JP, Bertrand HO (2005) J Med Chem 48(7):2534–2547
Verdonk ML, Berdini V, Hartshorn MJ, Mooij WT, Murray CW, Taylor RD, Watson P (2004). J Chem Inf Comput Sci 44(3)
Kirchmair J, Markt P, Distinto S, Wolber G, Langer T (2008). J Comput Aided Mol Des 22(3–4)
Irwin JJ, Shoichet BK (2005) J Chem Inf Model 45(1):177–182
Jacobsson M, Lidén P, Stjernschantz E, Boström H, Norinder U (2003) J Med Chem 46(26):5781–5789
Lipinski CA, Lombardo F, Dominy BW, Feeney PJ (2001) Adv Drug Deliv Rev 46(1–3):1–3
Veber DF, Johnson SR, Cheng HY, Smith BR, Ward KW, Kopple KD (2002) J Med Chem 45(12):2615–2623
Ellman G, Courtney K, Andresjr V, Featherstone R (1961) Biochem Pharmacol 7(2):88–95
Venkatachalam CM, Jiang X, Oldfield T, Waldman M (2003) J Mol Graph Model 21(4):289–307
Tamura M, Nakao H, Yoshizaki H, Shiratsuchi M, Shigyo H, Yamada H, Ozawa T, Totsuka J, Hidaka H (2005) Biochim Biophys Acta 1754(1–2):1–2
Bemis GW, Murcko MA (1996) J Med Chem 39(15):2887–2893
Poornima CS, Dean PM (1995) J Comput Aided Mol Des 9:500–512
Poornima CS, Dean PM (1995) J Comput Aided Mol Des 9:513–520
Poornima CS, Dean PM (1995) J Comput Aided Mol Des 9:521–531
Rogers D, Hopfinger AJ (1994) J Chem Inf Comput Sci 34(4):854–866
Walters W, Namchuk M (2003) Nat Rev Drug Discov 2:259–266
Brian KS (2006) J Med Chem 49:7274–7277
Hasegawa K, Miyashita Y, Funatsu KJ (1997) Chem Inf Comput Sci 37(2):306–310
Acknowledgments
This project was sponsored by the Deanship of Scientific Research at the University of Jordan. The authors wish to thank the National Cancer Institute for freely providing hit compounds for experimental validation.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Abuhamdah, S., Habash, M. & Taha, M.O. Elaborate ligand-based modeling coupled with QSAR analysis and in silico screening reveal new potent acetylcholinesterase inhibitors. J Comput Aided Mol Des 27, 1075–1092 (2013). https://doi.org/10.1007/s10822-013-9699-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10822-013-9699-6