Abstract
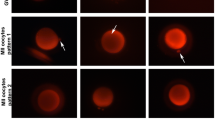
Refractile bodies are one of the main morphological abnormalities that can be observed in the cytoplasm of human oocytes. In the present studies the characteristics of refractile bodies and the relationship between the size of these structures and developmental competence of the affected oocytes and resulting embryos were examined. The refractile bodies were found to have yellow autofluorescence which was consistent with the typical autofluorescence of lipofuscin. Viewed by transmitted electron microscopy, the refractile bodies showed the conventional morphology of lipofuscin inclusions and consisted of a mixture of lipids and dense granule materials. Large refractile bodies (>5 μm) were positively stained by the Schmorl reaction and were considered to contain lipofuscin. These larger lipofuscin inclusions (>5 μm) were associated with significantly reduced fertilization and unfavorable blastocyst development.






Similar content being viewed by others
References
Daya S, Gunby J, Casper R. Oocyte morphology as a predictor of pregnancy for intracytoplasmic sperm injection. The 51st annual Clinical Meeting, The socirty of obstetricians and Gynecologists of Canada 1995; Abstr 013-REI, p. 47.
Alikani M, Palermo G, Adler A, Bertoli M, Blake M, Cohen J. Intracytoplasmic sperm injection in dysmorphic human oocytes. Zygote 1995;3:283–8.
Xia. Intracytoplasmic sperm injection: correlation of oocyte grade based on polar body, perivitelline space and cytoplasmic inclusions with fertilization rate and embryo quality. Hum Reprod 1997;12:1750–5.
De Sutter P, Dozortsev D, Qian C, Dhont M. Oocyte morphology does not correlate with fertilization rate and embryo quality after intracytoplasmic sperm injection. Hum Reprod 1996;11:595–7.
Balaban B, Urman B, Sertac A, Alatas C, Aksoy S, Mercan R. Oocyte morphology does not affect fertilization rate, embryo quality and implantation rate after intracytoplasmic sperm injection. Hum Reprod 1998;13:3431–3.
Veeck LL. Atlas of the human oocyte and early conceptus. Baltimore: Williams & Wilkins; 1991. p. 121–66.
Van Blerkom J. Occurrence and developmental consequences of aberrant cellular organization in meiotically mature human oocytes after exogenous ovarian hyperstimulation. J Electron Microsc Tech 1990;16:324–46.
Serhal PF, Ranieri DM, Kinis A, Marchant S, Davies M, Khadum IM. Oocyte morphology predicts outcome of intracytoplasmic sperm injection. Hum Reprod 1997;12:1267–70.
Hannover A. Mikroskopiske undersögelser af nervesystemet. Kgl. Danske Vidensk. Kabernes Selskobs Naturv Math Afh (Copenhagen) 1842;10:1–112.
Browne RM, Rippin JW. Autofluorescent granular cells in oral mucosal hyperplasias. Histopathology 1977;1:375–84.
Collins VP, Brunk UT. Characterization of residual bodies formed in phase II cultivated human glia cells. Mech Ageing Dev 1976;5:193–207.
Koneff H. Beiträge zur Kenntniss der Nervenzeilen den peripheren Ganglien. Mitt Naturforsch Gesellsch (Bern) 1886;44:13–4.
Glees P, Hasan M. Lipofuscin in neuronal aging and diseases. Norm Pathol Anat 1976;32:1–68.
Sohal RS, Donato Jr H. Effect of experimental prolongation of life span on lipofuscin content and lysosomal enzyme activity in the brain of the housefly, Musca domestica. J Gerontol 1979;34:489–96.
Munnell JF, Getty R. Rate of accumulation of cardiac lipofuscin in the aging canine. J Gerontol 1968;23:154–8.
Nakano M, Mizuno T, Gotoh S. Accumulation of cardiac lipofuscin in crab-eating monkeys (Macaca fasicularis): the same rate of lipofuscin accumulation in several species of primates. Mech Ageing Dev 1993;66:243–8.
Sohal RS, Brunk UT. Lipofuscin as an indicator of oxidative stress and aging. Adv Exp Med Biol 1989;266:17–29.
Gao G, Ollinger K, Brunk UT. Influence of intracellular glutathione concentration of lipofuscin accumulation in cultured neonatal rat cardiac myocytes. Free Radic Biol Med 1994;16:187–94.
Marzabadi MR, Sohal RS, Brunk UT. Mechanisms of lipofuscinogenesis: effect of the inhibition of lysosomal proteinases and lipases under varying concentrations of ambient oxygen in cultured rat neonatal myocardial cells. APMIS 1991;99:416–26.
Terman A. The effect of age on formation and elimination of autophagic vacuoles in mouse hepatocytes. Gerontology 1995;41:319–26.
Pearse AG. Hystochemistry: theoretical and applied. London: Churchill; 1968.
Cornillie FJ, Lauweryns JM. Phagocytotic and iron-storing capacities of stromal cells in the rat endometrium. A histochemical and ultrastructural study. Cell Tissue Res 1985;239:467–76.
Kuwamura M, Hattori R, Yamate J, Kotani T, Sasai K. Neuronal ceroid-lipofuscinosis and hydrocephalus in a chihuahua. J Small Anim Pract 2003;44:227–30.
Huang SZ, Luo YJ, Wang L, Cai KY. Effect of ginkgo biloba extract on livers in aged rats. World J Gastroenterol 2005;11:132–5.
Porta EA. Pigments in aging: an overview. Ann N Y Acad Sci 2002;959:57–65 (review).
Matsuura-Tokita K, Takeuchi M, Ichihara A, Mikuriya K, Nakano A. Live imaging of yeast Golgi cisternal maturation. Nature 2006;441:939–40.
Otsuki J, Okada A, Morimoto K, Nagai Y, Kubo H. The relationship between pregnancy outcome and smooth endoplasmic reticulum clusters in MII human oocytes. Hum Reprod 2004;19:1591–7.
Gao G, Ollinger K, Brunk UT. Influence of intracellular glutathione concentration of lipofuscin accumulation in cultured neonatal rat cardiac myocytes. Free Radic Biol Med 1994;16:187–94.
Marzabadi MR, Sohal RS, Brunk UT. Mechanisms of lipofuscinogenesis: effect of the inhibition of lysosomal proteinases and lipases under varying concentrations of ambient oxygen in cultured rat neonatal myocardial cells. APMIS 1991;99:416–26.
Ivy GO, Schottler F, Wenzel J, Baudry M, Lynch G: Inhibitors of lysosomal enzymes: accumulation of lipofuscin-like dense bodies in the brain. Science 1984;226:985–7.
Ivy GO, Kanai S, Ohta M, Smith G, Sato Y, Kobayashi M, et al. Lipofuscin-like substances accumulate rapidly in brain, retina and internal organs with cysteine protease inhibition. Adv Exp Med Biol 1989;266:31–45.
Ivy GO, Roopsingh R, Kanai S, Ohta M, Sato Y, Kitani K. Leupeptin causes an accumulation of lipofuscin-like substances and other signs of aging in kidneys of young rats: further evidence for the protease inhibitor model of aging. Ann N Y Acad Sci 1996;786:12–23.
Mitchell M, Armstrong DT, Robker RL, Norman RJ. Adipokines: implications for female fertility and obesity. Reproduction 2005;130:583–97.
Acknowledgements
The authors would like to thank Dr. Alexander Lopata for reading and commenting on this manuscript and Mr. Yoshihito Kondo at Tokyo Instruments for technical support on spectral image analysis.
Author information
Authors and Affiliations
Corresponding author
Additional information
Refractile bodies in human oocytes were found to contain lipofuscin. The largest lipofuscin inclusions (>5 μm) were associated with significantly reduced fertilization and unfavorable blastocyst development.
Rights and permissions
About this article
Cite this article
Otsuki, J., Nagai, Y. & Chiba, K. Lipofuscin bodies in human oocytes as an indicator of oocyte quality. J Assist Reprod Genet 24, 263–270 (2007). https://doi.org/10.1007/s10815-007-9130-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-007-9130-0