Abstract
Eutrophication is an increasing global threat to freshwater ecosystems. East Africa’s Lake Victoria has suffered from severe eutrophication in the past decades which is partly responsible for the dramatic decline in haplochromine cichlid species diversity. However, some zooplanktivorous and detritivorous haplochromine species recovered and shifted their diet towards macro invertebrates and fish. We used four formalin preserved cichlid species caught over the past 35 years to investigate whether stable isotopes of these fish are reflecting the dietary changes, habitat differences and if these isotopes can be used as indicators of eutrophication. We found that δ15N signatures mainly reflected dietary shifts to larger prey in all four haplochromine species. Shifts in δ13C signatures likely represented habitat differences and dietary changes. In addition, a shift to remarkably heavy δ13C signatures in 2011 was found for all four species which might infer increased primary production and thus eutrophication although more research is needed to confirm this hypothesis. The observed temporal changes confirm previous findings that preserved specimens can be used to trace historical changes in fish ecology and the aquatic environment. This highlights the need for continued sampling as this information could be of essence for reconstructing and predicting the effects of environmental changes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Eutrophication of freshwater ecosystems is increasingly common and is a major threat to biodiversity and to aquatic resource use by local human populations (Smith & Schindler, 2009). Most eutrophication assessment methods identified increased primary production as the immediate biological response to nutrient enrichment (Ferreira et al., 2011); and consequently, primary productivity has been recommended to be a sensitive and accurate indicator of eutrophication (Paerl et al., 2003; Andersen et al., 2006) with some exception Garmendia et al. (2013) and Smith (2007). Increased primary productivity and nutrient enrichment generally result in the preferential removal and depletion of lighter 12C leading to heavier δ13C signatures in aquatic food chains (Schelske & Hodell, 1991). Increased nitrogen pollution from runoff is reflected by heavier δ15N signatures while a high N demand by primary producers can favour N-fixing cyanobacteria and consequently lighter δ15N signatures (Peterson & Fry, 1987). Therefore, both carbon and nitrogen stable isotopes are sensitive to nutrient enrichment and increased primary productivity (Schelske & Hodell, 1991; Cabana & Rasmussen, 1996; Vander Zanden et al., 2005; Gu et al., 2006), and thus might be useful indicators of eutrophication.
Besides being used as indicators of primary productivity and of changes in basal signatures in food webs, stable isotopes are commonly used to function as estimators of trophic position and carbon flow in aquatic food webs (Peterson & Fry, 1987; Post, 2002). The δ15N signatures of consumers are typically enriched with 3–4‰ with each trophic level while the δ13C signatures are similar or only slightly enriched (δ13C < 1‰) (Peterson & Fry, 1987; Vander Zanden & Rasmussen, 2001). Stable isotopes can also provide information on the habitat of aquatic species. In general, limnetic phytoplankton photosynthesis results in lighter δ13C signatures compared to heavier δ13C signatures produced by benthic algae photosynthesizing within a boundary layer (France, 1995; Hecky & Hesslein, 1995). This phenomenon makes it possible to infer whether the prey of primary consumers has a benthic, littoral or limnetic origin (Hecky & Hesslein, 1995; Vander Zanden & Rasmussen, 1999). Stable isotopes of primary consumers are also related to the habitat gradient, with light δ13C and heavy δ15N signatures in profundal habitats and vice versa in littoral habitats (Vander Zanden & Rasmussen, 1999).
Lake Victoria has suffered from severe eutrophication in the past decades, and the shallow, inshore habitats especially have high algal biomasses and a high carbon demand by photosynthesis (Ramlal et al., 2001; Hecky et al., 2010). Based on paleolimnological analyses, changes in lower food web organisms began as early as the 1940s but accelerated dramatically through the 1960s and 1970s (Verschuren et al., 2002; Hecky et al., 2010). From the 1980s onwards, several studies have shown increased nitrogen and phosphorus loadings in the lake coinciding with decreased water transparency and oxygen levels (Mugidde, 1993; Hecky et al., 1994, 2010; Seehausen et al., 1997a; Verschuren et al., 2002). The eutrophication is thought to be caused by agricultural malpractices, urbanization and deforestation, although recent studies have suggested that climatic variability and wind stress played a crucial role as well (Kolding et al., 2008; Hecky et al., 2010; van Rijssel, 2014). Most of the studies reporting eutrophication focussed on the northern part of the lake (Hecky, 1993; Mugidde, 1993; Mugidde et al., 2003) and because of the lack of regular and consistent measurements of biological productivity, paleolimnological analysis was used to provide more continuous analysis of historical changes in the ecosystem.
For the Mwanza Gulf, located in the southern part of the lake, even less data on productivity are available (Akiyama et al., 1977; Shayo et al., 2011; Cornelissen et al., 2014), although other environmental variables such as dissolved oxygen levels and Secchi depth data have been measured on a fairly regular basis in the last four decades (van Rijssel, 2014). In addition, the Lake Victoria biodiversity crisis has been well documented for the Mwanza Gulf from the 1970s onwards (Witte et al., 2007). In the 1980s, the introduced Nile perch, Lates niloticus, population boomed. The Nile perch is thought to have contributed to the eutrophication of Lake Victoria as well by accelerating and subsidizing productivity of the ecosystem through high turnover of fish biomass and consequently more rapid recycling of nutrients (Kolding et al., 2008). Together with the eutrophication, the Nile perch boom caused a major decline of cichlid species (Witte et al., 1992; Seehausen et al., 1997a; Goudswaard et al., 2008). However, during the 1990s, some cichlid species, especially zooplanktivores and detritivores, recovered (Seehausen et al., 1997b; Witte et al., 2007; Kishe-Machumu et al., 2015) and shifted their diet towards macroinvertebrates and fish (Van Oijen & Witte, 1996 Katunzi et al., 2003; Kishe-Machumu et al., 2008; van Rijssel et al., 2015). With the use of formalin-preserved cichlid specimens collected over the past 35 years, we demonstrated that the recovered species showed morphological adaptive responses to the environmental changes (Witte et al., 2008; Van der Meer et al., 2012; Van Rijssel & Witte, 2013; van Rijssel et al., 2015).
Here, we use these unique cichlid museum specimens selected at triennial time intervals from 1978 onwards, to test how the environmental and ecological changes might be reflected in the C and N stable isotopes of these fish and if they can be used as indicators of eutrophication. In addition, we investigated whether habitat and seasonal changes were reflected in these isotopes as not all fish were caught at the exact same location and period on the research transect.
For this study, we used two closely related zooplanktivorous species (abbreviations of species in parentheses) Haplochromis (Yssichromis) pyrrhocephalusWitte & Witte-Maas 1987(pyr), H.(Y.) laparogramma Greenwood & Gee 1969 (lap), the zooplankti/insectivorous species H. tanaos van Oijen 1996 (tan) and the mollusci/detritivorous species Platytaeniodus degeniBoulenger 1906(deg). Dietary gut content analyses revealed that the species pyr and lap shifted their diet towards large macroinvertebrates such as aquatic insects, shrimps and molluscs as well as to fish during the 1990s, but reverted their diet (partly) back to zooplankton in the 2000s which is their original diet (Katunzi et al., 2003; Kishe-Machumu, 2012; van Rijssel et al., 2015). These two species also extended their habitat to shallower waters (Seehausen et al., 1997b; Kishe-Machumu et al., 2015). The species tan and deg both showed the most pronounced diet changes towards macroinvertebrates and fish during the 2000s and have extended their habitat to deeper waters (Van Oijen & Witte, 1996; Seehausen et al., 1997b; Kishe-Machumu et al., 2015; van Rijssel et al., 2015).
There are no substantial changes over time in the sedimentary δ15N of the lake based on results from three sediment cores from various locations in northern Lake Victoria (R. E. Hecky, unpubl. data). Therefore, we expect the dietary changes to be reflected in the δ15N signatures of the cichlids as was found by Kishe-Machumu et al. (this issue). However, the response of δ15N in fish muscle could be more complex as shifts in basal signature in phytoplankton will be additive to possible shifts in diet to prey which might be lighter or heavier in δ15N.
The eutrophication of the lake coincided with increased primary productivity, altered species composition and higher abundance of phytoplankton in the northern part (Hecky, 1993; Verschuren et al., 2002) as well as in the southern part of the lake (Cornelissen et al., 2014). The shift from diatoms to cyanobacterial phytoplankton dominance was accompanied with an increase of 2‰ in the (Suess corrected) δ13C of organic matter in sediment cores (Hecky et al., 2010). This probably occurred as the higher biomass of filamentous and colonial cyanobacteria raised the demand for CO2 relative to availability in this soft water lake (Ramlal et al., 2001) and also may have decreased isotopic fractionation by boundary layer effects in the larger filamentous and colonial cyanobacteria (Hecky & Hesslein, 1995). Therefore, we expect δ13C signatures may have shifted towards heavier values in these cichlids even without shifts in their diets, especially in inshore habitats. In any case, we hypothesized that changes in the environment and in the fish’s trophic level may be evident in the isotopic composition of the historical collection of haplochromine fishes from the Mwanza Gulf.
Methods
Fish collection
Most fish were collected from a research transect in the northern part of the Mwanza Gulf (6–14 m) on the southern coast of Lake Victoria. Fish were caught with a bottom trawler during the period 1978-2011. The species pyr and lap and were mainly caught above mud at station G (12–14 m) of the transect. Selected pyr specimens from 1987 were from Luanso Bay (Goldschmidt et al., 1993), a shallow bay (3–4 m) 10 kilometres south of the transect, as no pyr specimens caught on the transect in 1987 were preserved. The species tan and deg were mainly caught at sand/mud bottoms (Butimba and Kissenda Bay) at the opposite ends of the transect (Fig. 1). Fish were preserved in 4% formaldehyde (buffered with borax) and after shipment to Leiden, The Netherlands transferred to 70% ethanol and stored at theNaturalis Biodiversity Center. Species determination and distinction occurred for each individual in the field. F. Witte was responsible for the species re-determination for every preserved individual. A total of 273 male specimens (eight fish per year per species on average) was selected from the years 1978, 1981, 1984, 1987, 1991, 1993, 1999, 2001/02, 2006 and 2011 which is a selection from the same specimens used by van Rijssel & Witte (2013) and van Rijssel et al. (2015) (Table S1 in Electronic Supplementary Material).
Stable isotope analysis
From each fish, the right side of the epaxial muscle located dorsal of the lateral line was dissected after removal of the skin. These muscle tissue samples were freeze dried for 72 h and ground into fine powder. A subsample of 1.25 mg was placed into tin cups and shipped to the UC Davis Stable Isotope Facility for analysis. Stable isotope analysis of 13C and 15N was carried out with a PDZ Europa ANCA-GSL elemental analyzer interfaced to a PDZ Europa 20–20 continuous flow isotope ratio mass spectrometer (IRMS). The δ13C and δ15N values were expressed relative to international reference standards V-PDB (Vienna Pee Dee Belemnite) and air, respectively. The difference (δ) in isotopic ratio between the sample and standards was calculated as follows:
where R = 13CO2/12CO2 for δ13C or R = 15N2/14N2 for δ15N and values are expressed as ‰.
Glutamic acid, nylon and bovine liver which were similar in composition as the samples being used were used as standards. These standards were previously calibrated against NIST Standard Reference Materials such as IAEA-N1, IAEA-N2, IAEA-N3, USGS-40 and USGS-41.
Due to deforestation and fossil fuel burning which is naturally depleted in δ13C, atmospheric CO2 levels have been increasing while δ13C of CO2 has declined, especially over the past 35 years (Francey et al., 1999). This decrease in δ13C of atmospheric CO2 due to anthropogenic perturbations is known as the Suess effect (Keeling, 1979) and is most severe as the present day is approached (Verburg, 2007). As atmospheric and aquatic CO2 equilibrate rapidly in the upper mixed layer of lakes and oceans, it was necessary to apply a Suess correction in order to compare δ13C signatures of fish collected over the last 35 years according to the following formula:
with Y as year since 1700, as recommended by Verburg (2007). The Suess correction was subtracted from δ13C values of the years 1981–2011 with the smallest correction for 1981 (−0.07‰) and the largest correction for 2011 (−1.09‰).
Kishe-Machumu et al. (this issue) showed that formalin/ethanol preservation had a small but consistent effect on the stable isotopes of Lake Victoria cichlids. The preservation depleted δ13C with 0.66‰ and δ15N values increased on average with 0.34‰, which is consistent with directional shifts induced by formalin and ethanol preservation of fish reported in previous studies (Arrington & Winemiller, 2002; Sarakinos et al., 2002; Kelly et al., 2006; Gonzalez-Bergonzoni et al., 2015). So far, studies on long-term preservation effects of fish stable isotopes showed that these are independent of time (Kaehler & Pakhomov, 2001; Ogawa et al., 2001; Ponsard & Amlou, 1999; Sarakinos et al., 2002; Sweeting et al., 2004) which we assume is also the case for the cichlids used in this study. Although all fish of our study have been preserved, the same way over time and preservation effects are expected not to influence our results, we corrected the δ13C and δ15N values for preservation effects with +0.66‰ and −0.34‰, respectively (as reported by Kishe-Machumu et al., this issue) to approach more accurate stable isotope signatures.
Lipids are also known to influence δ13C analyses by fractionation which results in differences between δ13C values of lipids and other tissue like protein in a single organism (Deniro & Epstein, 1977; Mcconnaughey & Mcroy, 1979; Sweeting et al., 2006). Lipid content of tissue can be estimated by its C:N ratio (Mcconnaughey & Mcroy, 1979) after which a mathematical normalization technique can be applied to correct δ13C values for lipid content. By plotting δ13C values of each sample against its C:N ratio, we found a minor (slope = −0.02), but significant (P < 0.001) effect of lipids in our dataset with lower lipid contents in more recent years (Fig. S1). We corrected the dataset for lipid content using the formula:
which is proposed by Post et al. (2007) to use for aquatic organisms where δ13Cnormalized is the δ13C value normalized for lipid content and is comparable with δ13C after chemical lipid extraction. Fish samples without any lipids have a C:N ratio close to 3.0 (Kiljunen et al., 2006; Post et al., 2007). The average lipid content (C:N ratio) per sample in our study was fairly low (3.4, which is equal to 4.3% lipid), and it would therefore not be necessary to account for lipids (Post et al., 2007). Nonetheless, we corrected the samples for lipid content in order to adjust for the minor but significant trend found between δ13C and the lipid content.
In addition to generating stable isotope values from preserved cichlid specimens, we used stable isotope values of particulate organic matter (POM) to test how intra-lake variation in POM would influence our results. These POM stable isotope data were collected on a transect from Mwanza to Port Bell, Lake Victoria (see Fig. 2 in Hecky et al., 2010 for sampling transect). Every 20 km, surface water samples were taken from the trans-lake ferry MV Bukoba in October 1995. Additionally, water samples were taken from location V96-5MC in the middle of the lake, from Bugaia Island in the northern part of the lake in July 1995 and April 1996 (Campbell et al., 2003a; Hecky et al., 2010) and from offshore sites XL1, XL4, XL6-9 in May 1995 (Mugidde et al., 2003). Stable isotope signatures were derived from these samples using the methods of Hecky & Hesslein (1995) at the Freshwater Institute Laboratory (Winnipeg, Canada) and were also Suess corrected (Verburg, 2007).
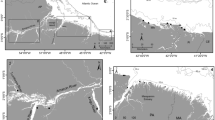
Arrow histograms of the angle of isotopic change between a the pristine and perturbed period, b the perturbed and recovery period for all four cichlid species. Arrows represent the direction and magnitude of isotopic change. The straight dashed line represents the mean vector of change; the curved dashed line on the rim represents the 95% confidence interval around the mean vector of change
Statistical analysis
We divided stable isotopes signatures into three different time periods according to van Rijssel et al. (2015). The pristine period (1978–1984), which is considered as the period before severe environmental changes; the perturbed period (1987–2002), which is considered as the period of severe environmental changes and changes in diet of the haplochromine cichlids; the recovery period (2006–2011), in which environmental changes seem less severe and some haplochromine species (partially) returned to their original diet.
To test our hypotheses on heavier δ13C and δ15N signatures over time, we applied circular statistics using Oriana 4.0 to quantify directional food web changes following Schmidt et al. (2007). We calculated the direction (angle of change) and length (magnitude of change) of both δ13C and δ15N combined per species over time (both for the three periods as for individual years). The directional change was calculated by considering the δ13C and δ15N values as x, y coordinates where delta y (15N) was divided by delta x (13C). These values were then converted into angles using the ATAN function in Excel. The direction (mean angle, µ) and the length (r) of the mean vector of all the species of the same period were calculated where (r) provides a unit of concentration of turning angles, r values close to 0 indicate that the directions are uniformly distributed, r values close to 1 indicate all angles are concentrated in the same direction. Angular standard deviation is given as measure of angular variance. We constructed arrow diagrams to visualize the direction and magnitude of each food web component. We used both Rayleigh’s test as Rao’s spacing test to assess whether the direction of the angles was uniformly distributed (null hypothesis).
Differences in stable isotopes per year were also tested with a One-way ANOVA. To test if standard length (SL) influences the stable isotopes of the fish, a Pearson correlation test was used after testing for normality with a Shapiro–Wilks test. In three out of four species (lap, pyr & tan), only five significant correlations between δ13C and SL were found within years. There were no significant correlations between δ15N and SL within years. Because there was no consistent trend and the correlations occurred in both positive and negative direction within a species, we decided not to correct for SL (Table S2).
To test if the number of different catch locations per year influenced the variation in stable isotopes, we correlated this number with the standard deviation (SD) of the stable isotopes per year (Table S3). We applied the same method to test for seasonal effects by correlating the number of catch dates with the SD of the stable isotopes.
Slope differences between stable isotope data of POM and preserved cichlid species were tested with an ANCOVA. All these statistical tests were performed with SPSS 20.
Results
Common isotopic responses per period
The direction of change in stable isotope signatures between the pristine and perturbed period differed between the four species (Fig. 2a; Table 1). The δ13C signatures of the two zooplanktivores pyr and lap changed unexpectedly towards lighter values while δ15N signatures increased as expected. The δ13C signatures of species tan and deg increased to heavier values and where δ15N signatures increased for deg, they unexpectedly decreased for tan (Fig. 2a). Arrow diagrams show that in the recovery period, stable isotope signatures of 3 out of 4 species (pyr, lap and deg) changed into the expected similar direction (Fig. 2b). This is supported by Raleigh’s and Rao’s spacing test which both indicate almost significant patterns of consistent change (0.10 > P > 0.05; Table 1) and a relatively high r (0.78). The δ13C signatures of pyr, lap and deg increased while those of tan remained similar. The δ15N signatures remained similar for pyr and lap while they increased for tan and deg (Fig. 2b).
Common isotopic responses per sampling year
All four species showed significant changes through time in δ13C (ANOVA, P < 0.001) and δ15N (ANOVA, P < 0.01; Figs. 3, 4; see Table S4 for F-statistics, degrees of freedom and P values). From 1978–1981, the three zooplanktivorous species shifted towards lighter δ13C values and heavier δ15N values while the species deg shifted in the opposite direction (Figs. 3a, 4a, b, c). From 1981 to 1984, pyr, lap and deg all shifted back towards heavier isotopic δ13C signatures, while δ15N values remained similar (µ = 0.86; Rayleigh’s test P = 0.10; Table 2, Figs. 3b,4a, b, d). From 1984 to 1987, these three species all showed a striking drop in δ15N signatures while δ13C values of lap and deg remained similar and those of pyr shifted towards heavier values (µ = 0.82; Rayleigh’s test P = 0.13; Table 2; Figs. 3c, 4a, b, d). It must be noted that specimens of pyr caught in 1987 came from the Luanso Bay, a shallow bay (3–4 m) 10 kilometres south of the transect which might be the cause of the relatively heavy δ13C values. In the periods 1987–1991, 1991–1993, 1993–1999 stable isotope signature of the two zooplanktivores pyr and lap both shifted towards lighter δ13C values and heavier δ15N values (Table 2; Figs. 3d, e, f, 4a, b) while from 1999 to 2002 a major shift towards heavier δ13C and lighter δ15N values occurred (µ = 1.0; Rayleigh’s test P = 0.14; Table 2; Figs. 3g, 4a, b). While the isotopic signatures of these two zooplanktivores continued to shift towards heavier δ13C values from 2002 to 2006, δ15N values of tan increased and δ13C values of deg shifted towards lighter values (Figs. 3h, 4). From 2006 to 2011, the isotopic signatures of all four species made a major shift towards remarkably heavy δ13C values, while δ15N values decreased for pyr, lap and tan during this period (µ = 0.95; Rayleigh’s test P = 0.02; Table 2, Figs. 3h, 4).
Arrow histograms of the angle of isotopic change between individual years for all four cichlid fish species. Arrows represent the direction and magnitude of isotopic change. The straight dashed line represents the mean vector of change, the curved dashed line on the rim represents the 95% confidence interval around the mean vector of change. Note that only in a, h, i all four species could be included in the histogram and that axes for magnitude differ for g and i
The Suess corrected δ13C and δ15N stable isotopes of the four cichlid species a H. laparogramma, b H. pyrrhocephalus, c H. tanaos and d P. degeni per year. Linear regression lines, their slopes, R-squared and P values are depicted for each species as a whole for comparison with particulate organic matter (POM) data, see discussion
Effect of catch location
Fish from multiple catch locations showed a higher catch within year variation in δ13C compared to fish caught in years with fewer catch locations (four different species combined, Spearman correlation, r = 0.422, P = 0.014). Since each catch location had a different depth, this means that fish caught in years with multiple catch locations were also caught from different depths. All four species showed positive (mostly non-significant) correlations between the number of catch locations per year and the SD of δ13C. There was one significant correlation for lap (r = 0.851, P = 0.002) and an almost significant correlation for deg (r = 0.732, P = 0.061) between the number of catch locations per year and the SD of δ13C (Table 3).
The relation between the number of catch locations and the amount of within year variation in δ15N was less clear and showed no significant correlations (Table 3).
The δ13C and δ15N values of POM exhibit an inverse relationship (Fig. 5, Pearson correlation, r = −0.50, P = 0.002) indicating intra-lake variation. This relationship shows that for every 1‰ increase in δ13C (from offshore to inshore), the δ15N decreases by 0.71‰ in POM. The species pyr, lap and tan seem to exhibit a similar trend with negative slopes of −0.40, −0.21 and −0.30, respectively (Figs. 4a–c). An ANCOVA on the slopes of these species and that of POM showed that the slope of lap differed significantly (P = 0.004) from POM but both slopes of pyr and tan did not (Table S5). The species deg showed a positive slope (0.41, Fig. 4d) which differed significantly from the slope of POM (P < 0.001; Table S5).
Stable isotopes of particulate organic matter (POM) collected from inshore and offshore stations along a transect from Mwanza in the south to Port Bell in the north of Lake Victoria in October 1995 and from location V96-5MC in the middle of the lake, from Bugaia Island in the northern part of the lake and from several offshore sites in 1995/1996 (see Campbell et al., 2003a; Mugidde et al., 2003; Hecky et al., 2010)
Effect of catch date
There was a significant positive correlation between the number of catch dates and the SD of δ13C per year for deg (r = 0.77, P = 0.043) and an almost significant positive correlation for lap (r = 0.574, P = 0.083). There were no significant correlations between the SD of δ15N and the number of catch dates per year (Table 3).
Discussion
Stable isotope changes through time
This study shows how dietary shifts are reflected in the stable isotopes of formalin-preserved Lake Victoria cichlids. The increase of δ15N values through time of all four species concurs with the reported shift in diet to larger prey for all four species. However, the hypothesized increase of δ13C did not occur until the 2000s, while we expected it to increase at the onset of eutrophication in 1987, the start of the perturbed period.
Diet-related stable isotope changes
Although the species shifted their diet already in 1987 (van Rijssel et al., 2015), there was no increase but a decrease in δ15N values in that year. Stomach and gut content analysis revealed that the diet of the zooplanktivores consisted for a large part of detritivorous shrimps and detritus (van Rijssel et al., 2015), which explains the low δ15N values. Campbell et al. (2003b) reported that Caridina (shrimps) had substantially lower δ15N and δ13C than zooplankton in Napoleon Gulf in northern Lake Victoria which is in agreement with our results. Though stomach and gut contents were not analysed for deg in that year, based on their low δ15N values and the dramatic increase of shrimps in the Mwanza Gulf during that time (Goudswaard et al., 2006; van Rijssel et al., 2015), it is likely that this species had shifted to a similar diet to that of the zooplanktivores.
Based on stomach and gut content analysis, the species tan shifted its diet in 1993 from zooplankton and insects to mainly insects and fish (Van Oijen & Witte, 1996). Although higher δ15N values might be expected with a shift to larger prey, tan already included quite a high percentage of insects in their diet (8% chironomids, 5% Chaoborus larvae and 24% other insects) before the environmental changes. Moreover, the aquatic insects and especially the decapod crustacean Caridina in Lake Victoria generally have lower δ15N values than zooplankton, although there are exceptions among the insects (Campbell et al., 2003b; Ojwang et al., 2004), which might explain the lack of δ15N increase in 1993 (Fig. 4; Table S4). In 2006, tan included even more fish in their diet than in 1993 (van Rijssel et al., 2015) which is reflected in the increase of δ15N values as well (Figs. 3, 4; Table S4). More consumption of aquatic insects might also explain the lower δ15N values of pyr compared to the closely related species lap from before the environmental changes. Haplochromis laparogramma was almost exclusively feeding on zooplankton during that time and pyr already included some chironomid larvae and insects next to their main prey zooplankton (Kishe-Machumu, 2012; van Rijssel et al., 2015) which might have lowered their δ15N values.
For one species (lap), we were able to perform a Pearson correlation test on the dietary contents with the stable isotopes from the same fish (data from van Rijssel et al., 2015). However, none of the averaged volume percentages of the different food types (zooplankton, phytoplankton, detritus, insects, shrimps or fish) gave a significant correlation with δ13C or δ15N in the fish through time (Table S6). The lack of correlation can be caused by three factors: (1) these fish seem to be quite opportunistic regarding their food types. The studied species shifted their diet from mainly small prey (zooplankton/detritus) to a highly diverse diet containing multiple food types such as insects, fish, shrimps, detritus and phytoplankton at the time that large macroinvertebrate numbers increased in their environment (van Rijssel et al., 2015). These lower food web organisms show a high variability in their stable isotope signatures (Campbell et al., 2003b) which might be reflected in the stable isotopes of the fish; (2) the stomach and gut contents only reflect what the fish has been eating that day (or night) and do not always have to reflect fish’s diet on the long term; (3) climatic variability seems to be affecting the mixing depths of the Mwanza Gulf (van Rijssel, 2014) which have an effect on the δ13C of particulate organic matter (POM) and fish and therefore interfere with stable isotope–food relationships. These three factors make direct dietary-stable isotope correlations hard to detect in these species.
Geographical variation
A higher number of catch locations resulted in a higher δ13C variation. Unfortunately, the dataset we used did not allow us to detect a general trend in offshore and inshore isotopes (heavier δ13C and lighter δ15N values inshore versus lighter δ13C and heavier δ15N offshore) as has been found by Hecky et al. (2010) and Mbabazi et al. (2010) in Lake Victoria and Lake Kyoga, respectively. However, these studies reported intra-lake variation on a large scale (from 1 to 150 km offshore) while our studied transect only covered 5 km. Our results show that the slopes between δ13C and δ15N in three out of four species have a similar direction compared to the slope of POM (with the slopes of pyr and tan showing no significant difference with that of POM). However, the slopes of these three species are less steep than that of POM, and so geographical variation can only partly explain the shifts in stable isotope signatures if we assume that the relationship for POM lake-wide applies to Mwanza Gulf. The positive slope of deg is in the opposite direction of the slope of POM so variation in deg isotopes can only be explained by a shift to isotopically heavier prey as both δ13C and δ15 N increase together from earlier to later years. The species pyr and lap did not extend their habitat to deeper water (as would be expected from the POM data and from the lighter δ13C signatures in these species through the 1980s and 1990s) but rather they occupied shallower water (Seehausen et al., 1997b; Kishe-Machumu et al., 2015). However, stomach and gut content analysis revealed a higher intake of chironomids, detritus and molluscs during the late 1980s and 1990s of both species (Katunzi et al., 2003; Kishe-Machumu 2012; van Rijssel et al., 2015), indicating a more benthic feeding behaviour during this period. In contrast, the species tan did extend its habitat from shallow bays to deeper open sublittoral areas. It is still unclear why some species have shifted from shallow to deeper waters while other species showed shifts in the opposite direction. Several causes of these habitat shifts have been suggested and most probably, a combination of these causalities has resulted in the habitat shifts. (1) A shift to the littoral habitat could be a response to heavy predation by Nile perch in the sublittoral zone; (2) the same shift could be a response to lower oxygen levels and reduced water transparency caused by increased eutrophication; (3) the shift from the littoral to the sublittoral habitat might be explained by competitive release and the opportunity to invade previously unoccupied spatial niches and; 4) ecological and morphological adaptive responses might have facilitated the habitat extension to deeper waters (Seehausen et al., 1997b; van der Meer et al., 2012; van Rijssel, 2014; Kishe-Machumu et al., 2015).
Although our dataset does not allow us to make within year comparisons, we suggest that geographical variation in δ13C isotopes might be present on a small scale like our research transect in cichlids. Specimens from the two closely related zooplanktivorous species pyr and lap from 1978 were caught all along the transect while fish of these species from 1981 were caught only at the deepest station of the transect, G (Table S1). Stomach and gut content analysis revealed these fish mainly fed on zooplankton and that there was no within species difference in volume percentages of this prey type before 1987 (van Rijssel et al., 2015).This is why we consider the shift towards lighter δ13C values of the two zooplanktivorous species in 1981 compared to 1978 (Figs. 3a, 4a, b) more likely to be the result of geographical variation than a change in diet over time. The observed trend for lighter δ13C values in deeper offshore water has been reported on a larger scale by Hecky et al. (2010). They attributed these lighter offshore δ13C values to a lower offshore algal (cyanobacteria) productivity and biomass compared to inshore. Although our research transect is only 5 km wide, the stable isotope data suggest that a similar relation might apply on a smaller scale to the Mwanza Gulf as well. This theory is supported by the findings of Kishe-Machumu et al. (this issue) who found heavier δ13C values at the shallow station J compared to deeper stations in the Mwanza Gulf for two haplochromine cichlid species (including H. pyrrhocephalus).
This geographical variation in stable isotopes suggests also that the zooplanktivorous open water species used in this study have a limited dispersal between stations along the transect. It is known that many cichlid species are restricted by bottom types, depths or parts of the water column but a virtual lack of horizontal migration that would be required to explain our data have not been reported for these open water species (Witte, 1981; Witte et al., 2007). On the other hand, these fish have extended their habitat to shallower depths in the past decades indicating that there must be some horizontal migration but probably less than previously thought (Seehausen et al., 1997b; Kishe-Machumu et al., 2015).
Seasonal variation
Primary producers are known to have within year temporal variation in both δ13C and δ15N stable isotopes (Cabana & Rasmussen, 1996; Post, 2002). Enriched (heavy) δ13C and decreased δ15N values of primary producers and primary consumers have been reported during periods of stratification in temperate lakes, but to our knowledge not in tropical lakes (Quay et al., 1986; Zohary et al., 1994; Hodell & Schelske 1998; Caroni et al., 2012). In addition, larger consumers such as fish have long tissue turnover rates (months to years, Hesslein et al., 1993) and thus are their isotopic signatures representative of their diet for longer periods of time (Post, 2002). This means that if there are small seasonal differences in the lower food web, they will be hard to detect, especially with the dataset used in this study where we were limited to museum material. The heavy δ13C and light δ15N values of 2011 found for lap, pyr and tan could, in theory, be considered as being a seasonal effect as these fish were all caught during the warmer wet season when vertical stratification of the water column is more likely than in the cool dry season and this may lead to different availability of food resources. In contrast, comparison of these isotopic signatures from 2011 with stable isotope values from fish caught during the wet season in the year 1999 shows that the latter actually had lighter δ13C and higher δ15N values, opposite from what one would expect if there is a strong seasonal effect. This leads us to believe that, based on our data, stable isotope signatures are a reflection of the fish’s diet and habitat rather than any possible seasonal effect. In addition, so far, no seasonal variation in the diet of Lake Victoria cichlids has been reported (Katunzi et al., 2003; Van Oijen & Witte, 1996; Kishe-Machumu, 2012; Kishe-Machumu et al., 2008; van Rijssel et al., 2015). Studies on seasonal variation of stable isotope signatures in Lake Victoria cichlids would provide definitive conclusions on this matter.
Signs of increased primary productivity?
Unexpectedly, the δ13C values in the studied zooplanktivorous species shifted to lighter values during the 1990s (the perturbed period) where heavier values were expected due to increased demand for CO2 and reduced isotopic fractionation resulting from the increased phytoplankton biomass (Hecky & Hesslein, 1995; Hecky et al., 2010). However, during the 2000s and especially in 2011, there is a remarkable shift towards heavier δ13C in all four species. We hypothesize that this might be the result of increased primary productivity by phytoplankton and evidence for continued eutrophication of the lake. Recently, Cornelissen et al. (2014) found that phytoplankton productivity has increased in the Mwanza Gulf compared to the 1970s (Akiyama et al., 1977). The increase of primary productivity and a basal change of phytoplankton stable isotope signatures could be reflected in the δ13C values of the fish when phytoplankton is (unintentionally) absorbed or ingested by the fish (or their prey), as has been found for several other fish species (especially during times of algal blooms, Christoffersen, 1996; Smith et al., 2008). In case of the zooplanktivorous species (which again include mainly zooplankton in 2006 and 2011, van Rijssel et al., 2015), the preyed upon zooplankton (mainly copepods) which should then feed upon cyanobacteria such as Microcystis and Anabaena and diatoms like Nitzschia which have replaced the original phytoplankton (mainly Aulacoseira[Melosira]) in the entire lake (Ochumba & Kibaara, 1989; Hecky, 1993; Kling et al., 2001; Verschuren et al., 2002) including the Mwanza Gulf (Sekadende et al., 2005; Cornelissen et al., 2014). However, grazing experiments indicated that Lake Victoria’s crustacean zooplankton (mainly cyclopoid copepods) do not control the cyanobacteria-dominated phytoplankton biomass (Branstrator et al., 1998; Lehman & Branstrator, 1993). In addition, other studies found cyanobacteria (Microcystis) to be toxic, nutritionally inadequate and able to suppress feeding in copepods (Demott & Moxter, 1991; Demott et al., 1991; Fulton & Paerl, 1987). On the other hand, there is a growing amount of evidence that copepods can grow and reproduce while feeding on toxic cyanobacteria (Koski et al., 2002; Reinikainen et al., 2002; Nascimento et al., 2008). In fact, several copepod species are known to (rapidly) adapt to increased cyanobacteria exposure enabling these zooplankters to feed upon the phytoplankton (Karjalainen et al., 2006; Colin & Dam, 2007; Mariani et al., 2013). Therefore, it is not improbable that the cyclopoid zooplankton (or cichlids) of the Mwanza Gulf partly feed upon the increased phytoplankton biomass that may have resulted in heavier δ13C values in our fish. A recent stable isotope study on zooplankton caught in the Mwanza Gulf in the wet season of 2011 (same location and period as our fish) showed the same heavy δ13C stable isotope values as for our fish (Cornelissen 2015), which supports the above-mentioned theory. Zooplankton grazing experiments on phytoplankton in the Mwanza Gulf would be needed to draw definitive conclusions.
Conclusion
With the use of a unique long-term formalin preserved sampling dataset, our study shows that stable isotope changes are reflecting dietary and habitat changes of four haplochromine species. In contrast, there does not seem to be a seasonal effect on the stable isotopes. Besides ecological changes, we suggest that the stable isotopes of these fish might be reflecting variation in primary production and varying degrees of eutrophication over the last several decades. This would imply that these haplochromines could serve as indicators of eutrophication.
The temporal variability of stable isotopes in these fishes confirms previous findings that museum specimens can be used to trace historical changes in fish ecology and the aquatic environment. This highlights the need for continued sampling of fish and as well as other aquatic organisms important to fish feeding to reconstruct and predict environmental changes in aquatic ecosystems.
References
Akiyama, T., A. A. Kajumulo & S. Olsen, 1977. Seasonal variations of plankton and physicochemical condition in Mwanza Gulf, Lake Victoria. Bulletin of Freshwater Fisheries Research Laboratory 27(2): 49–61.
Andersen, J. H., L. Schluter & G. Aertebjerg, 2006. Coastal eutrophication: recent developments in definitions and implications for monitoring strategies. Journal of Plankton Research 28(7): 621–628.
Arrington, D. A. & K. O. Winemiller, 2002. Preservation effects on stable isotope analysis of fish muscle. Transactions of the American Fisheries Society 131(2): 337–342.
Branstrator, D. K., J. T. Lehman & L. M. Ndawula, 1998. Zooplankton dynamics in Lake Victoria. In Johnson, T. C. & E. Odada (eds), Limnology, Climatology and Paleolimnology of the East African Lakes. Gordon and Breach, Amsterdam: 125–133.
Cabana, G. & J. B. Rasmussen, 1996. Comparison of aquatic food chains using nitrogen isotopes. Proceedings of the National Academy of Sciences of the United States of America 93(20): 10844–10847.
Campbell, L. M., R. E. Hecky, R. Muggide, D. G. Dixon & P. S. Ramlal, 2003a. Variation and distribution of total mercury in water, sediment and soil from northern Lake Victoria, East Africa. Biogeochemistry 65(2): 195–211.
Campbell, L. M., R. E. Hecky & S. B. Wandera, 2003b. Stable isotope analyses of food web structure and fish diet in Napoleon and Winam Gulfs, Lake Victoria, East Africa. Journal of Great Lakes Research 29: 243–257.
Caroni, R., G. Free, A. Visconti & M. Manca, 2012. Phytoplankton functional traits and seston stable isotopes signature: a functional-based approach in a deep, subalpine lake, Lake Maggiore (N. Italy). Journal of Limnology 71(1): 84–94.
Christoffersen, K., 1996. Ecological implications of cyanobacterial toxins in aquatic food webs. Phycologia 35(6S): 42–50.
Colin, S. P. & H. G. Dam, 2007. Comparison of the functional and numerical responses of resistant versus non-resistant populations of the copepod Acartia hudsonica fed the toxic dinoflagellate Alexandrium tamarense. Harmful Algae 6(6): 875–882.
Cornelissen, I. J. M., 2015. Eutrophication, Nile perch and food-web interactions in South-East Lake Victoria. PhD-Thesis, Wageningen University.
Cornelissen, I. J. M., G. M. Silsbe, J. A. J. Verreth, E. van Donk & L. A. J. Nagelkerke, 2014. Dynamics and limitations of phytoplankton biomass along a gradient in Mwanza Gulf, southern Lake Victoria (Tanzania). Freshwater Biology 59(1): 127–141.
Demott, W. R. & F. Moxter, 1991. Foraging on cyanobacteria by copepods - responses to chemical defenses and resource abundance. Ecology 72(5): 1820–1834.
Demott, W. R., Q. X. Zhang & W. W. Carmichael, 1991. Effects of toxic cyanobacteria and purified toxins on the survival and feeding of a copepod and 3 species of Daphnia. Limnology and Oceanography 36(7): 1346–1357.
Deniro, M. J. & S. Epstein, 1977. Mechanism of carbon isotope fractionation associated with lipid synthesis. Science 197(4300): 261–263.
Ferreira, J. G., J. H. Andersen, A. Borja, S. B. Bricker, J. Camp, M. C. da Silva, E. Garces, A. S. Heiskanen, C. Humborg, L. Ignatiades, C. Lancelot, A. Menesguen, P. Tett, N. Hoepffner & U. Claussen, 2011. Overview of eutrophication indicators to assess environmental status within the European Marine Strategy Framework Directive. Estuarine Coastal and Shelf Science 93(2): 117–131.
France, R. L., 1995. C13 Enrichment in benthic compared to planktonic algae - foodweb implications. Marine Ecology-Progress Series 124(1–3): 307–312.
Francey, R. J., C. E. Allison, D. M. Etheridge, C. M. Trudinger, I. G. Enting, M. Leuenberger, R. L. Langenfelds, E. Michel & L. P. Steele, 1999. A 1000-year high precision record of δ13C in atmospheric CO2. Tellus Series B-Chemical and Physical Meteorology 51(2): 170–193.
Fulton, R. S. & H. W. Paerl, 1987. Toxic and inhibitory effects of the blue-green alga Microcystis aeruginosa on herbivorous zooplankton. Journal of Plankton Research 9(5): 837–855.
Garmendia, M., A. Borja, J. Franco & M. Revilla, 2013. Phytoplankton composition indicators for the assessment of eutrophication in marine waters: present state and challenges within the European directives. Marine Pollution Bulletin 66(1–2): 7–16.
Goldschmidt, T., F. Witte & J. Wanink, 1993. Cascading effects of the introduced Nile perch on the detritivorous phytoplanktivorous species in the sublittoral areas of Lake Victoria. Conservation Biology 7(3): 686–700.
Gonzalez-Bergonzoni, I., N. Vidal, B. X. Wang, D. Ning, Z. W. Liu, E. Jeppesen & M. Meerhoff, 2015. General validation of formalin-preserved fish samples in food web studies using stable isotopes. Methods in Ecology and Evolution 6(3): 307–314.
Goudswaard, K. P. C., F. Witte & J. H. Wanink, 2006. The shrimp Caridina nilotica in Lake Victoria (East Africa), before and after the Nile perch increase. Hydrobiologia 563: 31–44.
Goudswaard, K. P. C., F. Witte & E. F. B. Katunzi, 2008. The invasion of an introduced predator, Nile perch (Lates niloticus, L.) in Lake Victoria (East Africa): chronology and causes. Environmental Biology of Fishes 81(2): 127–139.
Gu, B. H., A. D. Chapman & C. L. Schelske, 2006. Factors controlling seasonal variations in stable isotope composition of particulate organic matter in a soft water eutrophic lake. Limnology and Oceanography 51(6): 2837–2848.
Hecky, R. E., 1993. The eutrophication of Lake Victoria. Verhandlungen der Internationalen Vereinigung für Theoretische und Angewandte Limnologie 25: 39–48.
Hecky, R. E. & R. H. Hesslein, 1995. Contributions of benthic algae to lake food webs as revealed by stable isotope analysis. Journal of the North American Benthological Society 14(4): 631–653.
Hecky, R. E., F. W. B. Bugenyi, P. Ochumba, J. F. Talling, R. Mugidde, M. Gophen & L. Kaufman, 1994. Deoxygenation of the deep water of Lake Victoria, East Africa. Limnology and Oceanography 39(6): 1476–1481.
Hecky, R. E., R. Mugidde, P. S. Ramlal, M. R. Talbot & G. W. Kling, 2010. Multiple stressors cause rapid ecosystem change in Lake Victoria. Freshwater Biology 55: 19–42.
Hesslein, R. H., K. A. Hallard & P. Ramlal, 1993. Replacement of sulfur, carbon, and nitrogen in tissue of growing broad whitefish (Coregonus nasus) in response to a change in diet traced by delta-S-34, delta-C-13 and delta-N-15. Canadian Journal of Fisheries and Aquatic Sciences 50(10): 2071–2076.
Hodell, D. A. & C. L. Schelske, 1998. Production, sedimentation, and isotopic composition of organic matter in Lake Ontario. Limnology and Oceanography 43(2): 200–214.
Kaehler, S. & E. A. Pakhomov, 2001. Effects of storage and preservation on the delta C-13 and delta N-15 signatures of selected marine organisms. Marine Ecology Progress Series 219: 299–304.
Karjalainen, M., B. Kozlowsky-Suzuki, M. Lehtiniemi, J. Engstrom-Ost, H. Kankaanpaa & M. Viitasalo, 2006. Nodularin accumulation during cyanobacterial blooms and experimental depuration in zooplankton. Marine Biology 148(4): 683–691.
Katunzi, E. F. B., J. Zoutendijk, T. Goldschmidt, J. H. Wanink & F. Witte, 2003. Lost zooplanktivorous cichlid from Lake Victoria reappears with a new trade. Ecology of Freshwater Fish 12(4): 237–240.
Keeling, C. D., 1979. The Suess effect: 13Carbon–14Carbon interrelations. Environment International 2: 229–300.
Kelly, B., J. B. Dempson & M. Power, 2006. The effects of preservation on fish tissue stable isotope signatures. Journal of Fish Biology 69(6): 1595–1611.
Kiljunen, M., J. Grey, T. Sinisalo, C. Harrod, H. Immonen & R. I. Jones, 2006. A revised model for lipid-normalizing delta C-13 values from aquatic organisms, with implications for isotope mixing models. Journal of Applied Ecology 43(6): 1213–1222.
Kishe-Machumu, M. A., 2012. Inter-guild differences and possible causes of the recovery of cichlid species in Lake Victoria, Tanzania PhD thesis, Leiden University.
Kishe-Machumu, M. A., F. Witte & J. H. Wanink, 2008. Dietary shift in benthivorous cichlids after the ecological changes in Lake Victoria. Animal Biology 58(4): 401–417.
Kishe-Machumu, M. A., J. C. van Rijssel, J. H. Wanink & F. Witte, 2015. Differential recovery and spatial distribution of haplochromine cichlids in the Mwanza Gulf of Lake Victoria. Journal of Great Lakes Research 41: 454–462.
Kling, H. J., R. Mugidde & R. E. Hecky, 2001. Recent changes in the phytoplankton community of Lake Victoria in response to eutrophication. In Munawar, M. & R. E. Hecky (eds), The Great Lakes of the World (GLOW) - Food Web, Health and Integrity. Backhuys Publishers, Leiden: 47–65.
Kolding, J. P., P. Van Zwieten, O. C. Mkumbo, G. Silsbe & R. E. Hecky, 2008. Are the Lake Victoria fisheries threatened by exploitation or eutrophication? Towards an ecosystem based approach to management. In Bianchi, G. & H. R. Skjodal (eds), The Ecosystem Approach to Fisheries. Cab International, Wallingford: 309–354.
Koski, M., K. Schmidt, J. Engstrom-Ost, M. Viitasalo, S. Jonasdottir, S. Repka & K. Sivonen, 2002. Calanoid copepods feed and produce eggs in the presence of toxic cyanobacteria Nodularia spumigena. Limnology and Oceanography 47(3): 878–885.
Lehman, J. T. & D. K. Branstrator, 1993. Effects of nutrients and grazing on the phytoplankton of Lake Victoria. Verhandlungen der Internationalen Verein Limnologie 25: 846–849.
Mariani, P., K. H. Andersen, A. W. Visser, A. D. Barton & T. Kiorboe, 2013. Control of plankton seasonal succession by adaptive grazing. Limnology and Oceanography 58(1): 173–184.
Mbabazi, D., B. Makanga, F. Orach-Meza, R. E. Hecky, J. S. Balirwa, R. Ogutu-Ohwayo, P. Verburg, L. Chapman & E. Muhumuza, 2010. Intra-lake stable isotope ratio variation in selected fish species and their possible carbon sources in Lake Kyoga (Uganda): implications for aquatic food web studies. African Journal of Ecology 48(3): 667–675.
Mcconnaughey, T. & C. P. Mcroy, 1979. Food-web structure and the fractionation of carbon isotopes in the Bering Sea. Marine Biology 53(3): 257–262.
Mugidde, R., 1993. The increase in phytoplankton primary productivity and biomass in Lake Victoria (Uganda). Verhandlungen der Internationalen Vereinigung für Theoretische und Angewandte Limnologie 25: 846–849.
Mugidde, R., R. E. Hecky, L. L. Hendzel & W. D. Taylor, 2003. Pelagic nitrogen fixation in Lake Victoria (East Africa). Journal of Great Lakes Research 29: 76–88.
Nascimento, F. J. A., A. M. L. Karlson & R. Elmgren, 2008. Settling blooms of filamentous cyanobacteria as food for meiofauna assemblages. Limnology and Oceanography 53(6): 2636–2643.
Ochumba, P. B. O. & D. I. Kibaara, 1989. Observations on blue-green algal blooms in the open waters of Lake Victoria. Kenya. African Journal of Ecology 27(1): 23–34.
Ogawa, N. O., T. Koitabashi, H. Oda, T. Nakamura, N. Ohkouchi & E. Wada, 2001. Fluctuations of nitrogen isotope ratio of gobiid fish (Isaza) specimens and sediments in Lake Biwa, Japan, during the 20th century. Limnology and Oceanography 46(5): 1228–1236.
Ojwang, W. O., L. Kaufman, A. A. Asila, S. Agembe & B. Michener, 2004. Isotopic evidence of functional overlap amongst the resilient pelagic fishes of Lake Victoria, Kenya. Hydrobiologia 529(1): 27–35.
Paerl, H. W., L. M. Valdes, J. L. Pinckney, M. F. Piehler, J. Dyble & P. H. Moisander, 2003. Phytoplankton photopigments as indicators of estuarine and coastal eutrophication. Bioscience 53(10): 953–964.
Peterson, B. J. & B. Fry, 1987. Stable isotopes in ecosystem studies. Annual Review of Ecology and Systematics 18: 293–320.
Ponsard, S. & M. Amlou, 1999. Effects of several preservation methods on the isotopic content of Drosophila samples. Comptes Rendus De L Academie Des Sciences Serie Iii-Sciences De La Vie-Life Sciences 322(1): 35–41.
Post, D. M., 2002. Using stable isotopes to estimate trophic position: models, methods, and assumptions. Ecology 83(3): 703–718.
Post, D. M., C. A. Layman, D. A. Arrington, G. Takimoto, J. Quattrochi & C. G. Montana, 2007. Getting to the fat of the matter: models, methods and assumptions for dealing with lipids in stable isotope analyses. Oecologia 152(1): 179–189.
Quay, P. D., S. R. Emerson, B. M. Quay & A. H. Devol, 1986. The carbon cycle for Lake Washington - a stable isotope study. Limnology and Oceanography 31(3): 596–611.
Ramlal, P. S., G. W. Kling, L. M. Ndawula, R. E. Hecky & H. J. Kling, 2001. Diurnal fluctuations in PCO2, DIC, oxygen and nutrients at inshore sites in Lake Victoria, Uganda. In Munawar, M. & R. E. Hecky (eds), The Great Lakes of the World (GLOW) - Food Web, Health and Integrity. Backhuys Publishers, Leiden: 67–82.
Reinikainen, M., F. Lindvall, J. A. O. Meriluoto, S. Repka, K. Sivonen, L. Spoof & M. Wahlsten, 2002. Effects of dissolved cyanobacterial toxins on the survival and egg hatching of estuarine calanoid copepods. Marine Biology 140(3): 577–583.
Sarakinos, H. C., M. L. Johnson & M. J. Vander Zanden, 2002. A synthesis of tissue-preservation effects on carbon and nitrogen stable isotope signatures. Canadian Journal of Zoology-Revue Canadienne De Zoologie 80(2): 381–387.
Schelske, C. L. & D. A. Hodell, 1991. Recent changes in productivity and climate of Lake Ontario detected by isotopic analysis of sediments. Limnology and Oceanography 36(5): 961–975.
Schmidt, S. N., J. D. Olden, C. T. Solomon & M. J. Vander Zanden, 2007. Quantitative approaches to the analysis of stable isotope food web data. Ecology 88(11): 2793–2802.
Seehausen, O., J. J. M. van Alphen & F. Witte, 1997a. Cichlid fish diversity threatened by eutrophication that curbs sexual selection. Science 277(5333): 1808–1811.
Seehausen, O., F. Witte, E. F. Katunzi, J. Smits & N. Bouton, 1997b. Patterns of the remnant cichlid fauna in southern Lake Victoria. Conservation Biology 11(4): 890–904.
Sekadende, B. C., T. J. Lyimo & R. Kurmayer, 2005. Microcystin production by cyanobacteria in the Mwanza Gulf (Lake Victoria, Tanzania). Hydrobiologia 543: 299–304.
Shayo, S. D., C. Lugomela & J. F. Machiwa, 2011. Influence of land use patterns on some limnological characteristics in the south-eastern part of Lake Victoria. Tanzania. Aquatic Ecosystem Health & Management 14(3): 246–251.
Smith, J. L., G. L. Boyer & P. V. Zimba, 2008. A review of cyanobacterial odorous and bioactive metabolites: impacts and management alternatives in aquaculture. Aquaculture 280(1–4): 5–20.
Smith, V. H., 2007. Using primary productivity as an index of coastal eutrophication: the units of measurement matter. Journal of Plankton Research 29(1): 1–6.
Smith, V. H. & D. W. Schindler, 2009. Eutrophication science: where do we go from here? Trends in Ecology & Evolution 24(4): 201–207.
Sweeting, C. J., N. V. C. Polunin & S. Jennings, 2004. Tissue and fixative dependent shifts of delta C-13 and delta N-15 in preserved ecological material. Rapid Communications in Mass Spectrometry 18(21): 2587–2592.
Sweeting, C. J., N. V. C. Polunin & S. Jennings, 2006. Effects of chemical lipid extraction and arithmetic lipid correction on stable isotope ratios of fish tissues. Rapid Communications in Mass Spectrometry 20(4): 595–601.
Van der Meer, H. J., J. C. van Rijssel, L. C. Wagenaar & F. Witte, 2012. Photopic adaptations to a changing environment in two Lake Victoria cichlids. Biological Journal of the Linnean Society 106(2): 328–341.
Van Oijen, M. J. P. & F. Witte, 1996. Taxonomical and ecological description of a species complex of zooplanktivorous and insectivorous cichlids from Lake Victoria. Zool Verh Leiden 302: 1–56.
van Rijssel, J. C., 2014. Adaptive responses to environmental changes in Lake Victoria cichlids. PhD-Thesis, Leiden University.
van Rijssel, J. C., E. S. Hoogwater, M. A. Kishe-Machumu, E. van Reenen, K. V. Spits, R. C. van der Stelt, J. H. Wanink & F. Witte, 2015. Fast adaptive responses in the oral jaw of Lake Victoria cichlids. Evolution 69(1): 179–189.
Van Rijssel, J. C. & F. Witte, 2013. Adaptive responses in resurgent Lake Victoria cichlids over the past 30 years. Evolutionary Ecology 27(2): 253–267.
Vander Zanden, M. J. & J. B. Rasmussen, 1999. Primary consumer δ13C and δ15N and the trophic position of aquatic consumers. Ecology 80(4): 1395–1404.
Vander Zanden, M. J. & J. B. Rasmussen, 2001. Variation in δ15N and δ13C trophic fractionation: implications for aquatic food web studies. Limnology and Oceanography 46(8): 2061–2066.
Vander Zanden, M. J., Y. Vadeboncoeur, M. W. Diebel & E. Jeppesen, 2005. Primary consumer stable nitrogen isotones as indicators of nutrient source. Environmental Science & Technology 39(19): 7509–7515.
Verburg, P., 2007. The need to correct for the Suess effect in the application of delta C-13 in sediment of autotrophic Lake Tanganyika, as a productivity proxy in the Anthropocene. Journal of Paleolimnology 37(4): 591–602.
Verschuren, D., T. C. Johnson, H. J. Kling, D. N. Edgington, P. R. Leavitt, E. T. Brown, M. R. Talbot & R. E. Hecky, 2002. History and timing of human impact on Lake Victoria, East Africa. Proceedings of the Royal Society Biological Sciences Series B 269(1488): 289–294.
Witte, F., 1981. Initial results of the ecological survey of the haplochromine cichlid fishes from the Mwanza Gulf of Lake Victoria (Tanzania) - breeding patterns, trophic and species distribution - with recommendations for commercial trawl-fishery. Netherlands Journal of Zoology 31(1): 175–202.
Witte, F., T. Goldschmidt, J. H. Wanink, M. J. P. van Oijen, K. P. C. Goudswaard, E. Witte-maas & N. Bouton, 1992. The destruction of an endemic species flock - quantitative data on the decline of the haplochromine cichlids of Lake Victoria. Environmental Biology of Fishes 34(1): 1–28.
Witte, F., J. H. Wanink & M. A. Kishe, 2007. Species distinction and the biodiversity crisis in Lake Victoria. Transactions of the American Fisheries Society 136: 1146–1159.
Witte, F., M. Welten, M. Heemskerk, I. van der Stap, L. Ham, H. Rutjes & J. Wanink, 2008. Major morphological changes in a Lake Victoria cichlid fish within two decades. Biological Journal of the Linnean Society 94(1): 41–52.
Zohary, T., J. Erez, M. Gophen, I. Bermanfrank & M. Stiller, 1994. Seasonality of stable carbon isotopes within the pelagic food web of Lake Kinneret. Limnology and Oceanography 39(5): 1030–1043.
Acknowledgments
We are thankful for our colleagues from the Haplochromis Ecological Survey Team (HEST) and the Tanzania Fisheries Research Institute (TAFIRI) for support and co-operation during the fieldwork. We also like to thank the two anonymous reviewers for helpful comments and suggestions for improvement of the manuscript. The research and fieldwork was financially supported by The Netherlands Organization for Scientific Research (NWO Grant: ALW1PJ/07030), The Netherlands Foundation for the Advancement of Tropical Research (WOTRO Grants: W87-129, W87-161, W87-189, W84-282, W84-488, WB84-587), by the Section of Research and Technology of the Netherlands’ Ministry of Development Co-operation, the Netherlands Organization for International Cooperation in Higher Education (NUFFIC), the International Foundation for Sciences (IFS) and the Schure Beijerinck-Popping Fonds.
Author information
Authors and Affiliations
Corresponding author
Additional information
Guest editors: S. Koblmüller, R. C. Albertson, M. J. Genner, K. M. Sefc & T. Takahashi / Advances in Cichlid Research II: Behavior, Ecology and Evolutionary Biology
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
van Rijssel, J.C., Hecky, R.E., Kishe-Machumu, M.A. et al. Changing ecology of Lake Victoria cichlids and their environment: evidence from C13 and N15 analyses. Hydrobiologia 791, 175–191 (2017). https://doi.org/10.1007/s10750-016-2790-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-016-2790-y