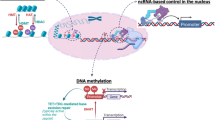
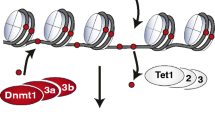
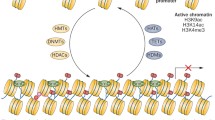
Abstract
With the impressive advancement in high-throughput ‘omics’ technologies over the past two decades, epigenetic mechanisms have emerged as the regulatory interface between the genome and environmental factors. These mechanisms include DNA methylation, histone modifications, ATP-dependent chromatin remodeling and RNA-based mechanisms. Their highly interdependent and coordinated action modulates the chromatin structure controlling access of the transcription machinery and thereby regulating expression of target genes. Given the rather limited proliferative capability of human cardiomyocytes, epigenetic regulation appears to play a particularly important role in the myocardium. The highly dynamic nature of the epigenome allows the heart to adapt to environmental challenges and to respond quickly and properly to cardiac stress. It is now becoming evident that histone-modifying and chromatin-remodeling enzymes as well as numerous non-coding RNAs play critical roles in cardiac development and function, while their dysregulation contributes to the onset and development of pathological cardiac remodeling culminating in HF. This review focuses on up-to-date knowledge about the epigenetic mechanisms and highlights their emerging role in the healthy and failing heart. Uncovering the determinants of epigenetic regulation holds great promise to accelerate the development of successful new diagnostic and therapeutic strategies in human cardiac disease.



Similar content being viewed by others
References
Marin-Garcia J, Akhmedov A, Rybin V, Moe GW (2014) Post-genomic cardiology, 2nd edn. Elsevier, Amsterdam, p 924
Nabel EG, Braunwald E (2012) A tale of coronary artery disease and myocardial infarction. N Engl J Med 366:54–63
Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD et al (2012) Executive summary: heart disease and stroke statistics—2012 update: a report from the American Heart Association. Circulation 125:188–197
Braunwald E (2013) Research advances in heart failure: a compendium. Circ Res 113:633–645
Heidenreich PA, Albert NM, Allen LA, Bluemke DA, Butler J et al (2013) Forecasting the impact of heart failure in the United States: a policy statement from the American Heart Association. Circ Heart Fail 6:606–619
Roger VL (2013) Epidemiology of heart failure. Circ Res 113:646–659
Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr et al (2013) 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 62:e147–e239
Mudd JO, Kass DA (2008) Tackling heart failure in the twenty-first century. Nature 451:919–928
Shah AM, Mann DL (2011) In search of new therapeutic targets and strategies for heart failure: recent advances in basic science. Lancet 378:704–712
McMurray JJ, Pfeffer MA (2005) Heart failure. Lancet 365:1877–1889
Monnet E, Chachques JC (2005) Animal models of heart failure: what is new? Ann Thorac Surg 79:1445–1453
Klocke R, Tian W, Kuhlmann MT, Nikol S (2007) Surgical animal models of heart failure related to coronary heart disease. Cardiovasc Res 74:29–38
Zornoff LA, Paiva SA, Duarte DR, Spadaro J (2009) Ventricular remodeling after myocardial infarction: concepts and clinical implications. Arq Bras Cardiol 92:150–164
McMurray JJ (2010) Clinical practice. Systolic heart failure. N Engl J Med 362:228–238
Neubauer S (2007) The failing heart—an engine out of fuel. N Engl J Med 356:1140–1151
Ingwall JS (2009) Energy metabolism in heart failure and remodelling. Cardiovasc Res 81:412–419
Abel ED, Doenst T (2011) Mitochondrial adaptations to physiological vs. pathological cardiac hypertrophy. Cardiovasc Res 90:234–242
Azevedo PS, Minicucci MF, Santos PP, Paiva SA, Zornoff LA (2013) Energy metabolism in cardiac remodeling and heart failure. Cardiol Rev 21:135–140
Giordano FJ (2005) Oxygen, oxidative stress, hypoxia, and heart failure. J Clin Invest 115:500–508
Maack C, Bohm M (2011) Targeting mitochondrial oxidative stress in heart failure throttling the afterburner. J Am Coll Cardiol 58:83–86
Santos CX, Anilkumar N, Zhang M, Brewer AC, Shah AM (2011) Redox signaling in cardiac myocytes. Free Radic Biol Med 50:777–793
Bers DM (2006) Altered cardiac myocyte Ca regulation in heart failure. Physiology (Bethesda) 21:380–387
Neef S, Maier LS (2013) Novel aspects of excitation-contraction coupling in heart failure. Basic Res Cardiol 108:360
Dorn GW 2nd (2009) Apoptotic and non-apoptotic programmed cardiomyocyte death in ventricular remodelling. Cardiovasc Res 81:465–473
Whelan RS, Kaplinskiy V, Kitsis RN (2010) Cell death in the pathogenesis of heart disease: mechanisms and significance. Annu Rev Physiol 72:19–44
Heidecker B, Kasper EK, Wittstein IS, Champion HC, Breton E et al (2008) Transcriptomic biomarkers for individual risk assessment in new-onset heart failure. Circulation 118:238–246
Creemers EE, Wilde AA, Pinto YM (2011) Heart failure: advances through genomics. Nat Rev Genet 12:357–362
Kurabayashi M, Shibasaki Y, Komuro I, Tsuchimochi H, Yazaki Y (1990) The myosin gene switching in human cardiac hypertrophy. Jpn Circ J 54:1192–1205
Herron TJ, McDonald KS (2002) Small amounts of alpha-myosin heavy chain isoform expression significantly increase power output of rat cardiac myocyte fragments. Circ Res 90:1150–1152
Olson EN, Backs J, McKinsey TA (2006) Control of cardiac hypertrophy and heart failure by histone acetylation/deacetylation. Novartis Found Symp 274:3–12 discussion 13–19, 152–155, 272–156
Holliday R (2006) Epigenetics: a historical overview. Epigenetics 1:76–80
Bird A (2007) Perceptions of epigenetics. Nature 447:396–398
Feinberg AP (2007) Phenotypic plasticity and the epigenetics of human disease. Nature 447:433–440
Baccarelli A, Bollati V (2009) Epigenetics and environmental chemicals. Curr Opin Pediatr 21:243–251
Feil R, Fraga MF (2011) Epigenetics and the environment: emerging patterns and implications. Nat Rev Genet 13:97–109
Rakyan VK, Hildmann T, Novik KL, Lewin J, Tost J et al (2004) DNA methylation profiling of the human major histocompatibility complex: a pilot study for the human epigenome project. PLoS Biol 2:e405
Abbott A (2010) Project set to map marks on genome. Nature 463:596–597
Rakyan VK, Down TA, Balding DJ, Beck S (2011) Epigenome-wide association studies for common human diseases. Nat Rev Genet 12:529–541
Baccarelli A, Rienstra M, Benjamin EJ (2010) Cardiovascular epigenetics: basic concepts and results from animal and human studies. Circ Cardiovasc Genet 3:567–573
Ordovas JM, Smith CE (2010) Epigenetics and cardiovascular disease. Nat Rev Cardiol 7:510–519
Handy DE, Castro R, Loscalzo J (2011) Epigenetic modifications: basic mechanisms and role in cardiovascular disease. Circulation 123:2145–2156
Movassagh M, Vujic A, Foo R (2011) Genome-wide DNA methylation in human heart failure. Epigenomics 3:103–109
Shirodkar AV, Marsden PA (2011) Epigenetics in cardiovascular disease. Curr Opin Cardiol 26:209–215
Chang CP, Bruneau BG (2012) Epigenetics and cardiovascular development. Annu Rev Physiol 74:41–68
Duygu B, Poels EM, da Costa Martins PA (2013) Genetics and epigenetics of arrhythmia and heart failure. Front Genet 4:219
Udali S, Guarini P, Moruzzi S, Choi SW, Friso S (2013) Cardiovascular epigenetics: from DNA methylation to microRNAs. Mol Aspects Med 34:883–901
Abi Khalil C (2014) The emerging role of epigenetics in cardiovascular disease. Ther Adv Chronic Dis 5:178–187
Cao DJ (2014) Epigenetic regulation and heart failure. Expert Rev Cardiovasc Ther 12:1087–1098
Ren B, Robert F, Wyrick JJ, Aparicio O, Jennings EG et al (2000) Genome-wide location and function of DNA binding proteins. Science 290:2306–2309
Kwon YS, Garcia-Bassets I, Hutt KR, Cheng CS, Jin M et al (2007) Sensitive ChIP-DSL technology reveals an extensive estrogen receptor alpha-binding program on human gene promoters. Proc Natl Acad Sci USA 104:4852–4857
Nielsen R, Pedersen TA, Hagenbeek D, Moulos P, Siersbaek R et al (2008) Genome-wide profiling of PPARgamma:RXR and RNA polymerase II occupancy reveals temporal activation of distinct metabolic pathways and changes in RXR dimer composition during adipogenesis. Genes Dev 22:2953–2967
Bock C, Tomazou EM, Brinkman AB, Muller F, Simmer F et al (2010) Quantitative comparison of genome-wide DNA methylation mapping technologies. Nat Biotechnol 28:1106–1114
Harris RA, Wang T, Coarfa C, Nagarajan RP, Hong C et al (2010) Comparison of sequencing-based methods to profile DNA methylation and identification of monoallelic epigenetic modifications. Nat Biotechnol 28:1097–1105
Ehrlich M, Gama-Sosa MA, Huang LH, Midgett RM, Kuo KC et al (1982) Amount and distribution of 5-methylcytosine in human DNA from different types of tissues of cells. Nucleic Acids Res 10:2709–2721
Bird A (2002) DNA methylation patterns and epigenetic memory. Genes Dev 16:6–21
Law JA, Jacobsen SE (2010) Establishing, maintaining and modifying DNA methylation patterns in plants and animals. Nat Rev Genet 11:204–220
Lister R, Pelizzola M, Dowen RH, Hawkins RD, Hon G et al (2009) Human DNA methylomes at base resolution show widespread epigenomic differences. Nature 462:315–322
Barres R, Osler ME, Yan J, Rune A, Fritz T et al (2009) Non-CpG methylation of the PGC-1alpha promoter through DNMT3B controls mitochondrial density. Cell Metab 10:189–198
Suzuki MM, Bird A (2008) DNA methylation landscapes: provocative insights from epigenomics. Nat Rev Genet 9:465–476
Illingworth RS, Bird AP (2009) CpG islands—’a rough guide’. FEBS Lett 583:1713–1720
Weber M, Hellmann I, Stadler MB, Ramos L, Paabo S et al (2007) Distribution, silencing potential and evolutionary impact of promoter DNA methylation in the human genome. Nat Genet 39:457–466
Shen L, Kondo Y, Guo Y, Zhang J, Zhang L et al (2007) Genome-wide profiling of DNA methylation reveals a class of normally methylated CpG island promoters. PLoS Genet 3:2023–2036
Cedar H, Bergman Y (2009) Linking DNA methylation and histone modification: patterns and paradigms. Nat Rev Genet 10:295–304
Irizarry RA, Ladd-Acosta C, Wen B, Wu Z, Montano C et al (2009) The human colon cancer methylome shows similar hypo- and hypermethylation at conserved tissue-specific CpG island shores. Nat Genet 41:178–186
Hendrich B, Tweedie S (2003) The methyl-CpG binding domain and the evolving role of DNA methylation in animals. Trends Genet 19:269–277
Nikitina T, Shi X, Ghosh RP, Horowitz-Scherer RA, Hansen JC et al (2007) Multiple modes of interaction between the methylated DNA binding protein MeCP2 and chromatin. Mol Cell Biol 27:864–877
Goll MG, Bestor TH (2005) Eukaryotic cytosine methyltransferases. Annu Rev Biochem 74:481–514
Cheng X, Blumenthal RM (2008) Mammalian DNA methyltransferases: a structural perspective. Structure 16:341–350
Reik W (2007) Stability and flexibility of epigenetic gene regulation in mammalian development. Nature 447:425–432
Sasaki H, Matsui Y (2008) Epigenetic events in mammalian germ-cell development: reprogramming and beyond. Nat Rev Genet 9:129–140
Leonhardt H, Page AW, Weier HU, Bestor TH (1992) A targeting sequence directs DNA methyltransferase to sites of DNA replication in mammalian nuclei. Cell 71:865–873
Kim JK, Samaranayake M, Pradhan S (2009) Epigenetic mechanisms in mammals. Cell Mol Life Sci 66:596–612
Ooi SK, Bestor TH (2008) The colorful history of active DNA demethylation. Cell 133:1145–1148
Bhutani N, Burns DM, Blau HM (2011) DNA demethylation dynamics. Cell 146:866–872
Olins DE, Olins AL (2003) Chromatin history: our view from the bridge. Nat Rev Mol Cell Biol 4:809–814
Kornberg RD, Lorch Y (1999) Twenty-five years of the nucleosome, fundamental particle of the eukaryote chromosome. Cell 98:285–294
Davey CA, Sargent DF, Luger K, Maeder AW, Richmond TJ (2002) Solvent mediated interactions in the structure of the nucleosome core particle at 1.9 a resolution. J Mol Biol 319:1097–1113
Richmond TJ, Davey CA (2003) The structure of DNA in the nucleosome core. Nature 423:145–150
Bell O, Tiwari VK, Thoma NH, Schubeler D (2011) Determinants and dynamics of genome accessibility. Nat Rev Genet 12:554–564
Kouzarides T (2007) Chromatin modifications and their function. Cell 128:693–705
Kacem S, Feil R (2009) Chromatin mechanisms in genomic imprinting. Mamm Genome 20:544–556
Young NL, Dimaggio PA, Garcia BA (2010) The significance, development and progress of high-throughput combinatorial histone code analysis. Cell Mol Life Sci 67:3983–4000
Hanover JA, Krause MW, Love DC (2012) Bittersweet memories: linking metabolism to epigenetics through O-GlcNAcylation. Nat Rev Mol Cell Biol 13:312–321
Bruneau BG (2010) Chromatin remodeling in heart development. Curr Opin Genet Dev 20:505–511
Vallaster M, Vallaster CD, Wu SM (2012) Epigenetic mechanisms in cardiac development and disease. Acta Biochim Biophys Sin (Shanghai) 44:92–102
Mahmoud SA, Poizat C (2013) Epigenetics and chromatin remodeling in adult cardiomyopathy. J Pathol 231:147–157
Mathiyalagan P, Keating ST, Du XJ, El-Osta A (2014) Chromatin modifications remodel cardiac gene expression. Cardiovasc Res 103:7–16
Dawson MA, Kouzarides T (2012) Cancer epigenetics: from mechanism to therapy. Cell 150:12–27
Jenuwein T, Allis CD (2001) Translating the histone code. Science 293:1074–1080
Wang GG, Allis CD, Chi P (2007) Chromatin remodeling and cancer, Part I: covalent histone modifications. Trends Mol Med 13:363–372
Campos EI, Reinberg D (2009) Histones: annotating chromatin. Annu Rev Genet 43:559–599
Bannister AJ, Kouzarides T (2011) Regulation of chromatin by histone modifications. Cell Res 21:381–395
Tan M, Luo H, Lee S, Jin F, Yang JS et al (2011) Identification of 67 histone marks and histone lysine crotonylation as a new type of histone modification. Cell 146:1016–1028
Kouzarides T (2007) SnapShot: histone-modifying enzymes. Cell 128:802
Yang XJ, Seto E (2007) HATs and HDACs: from structure, function and regulation to novel strategies for therapy and prevention. Oncogene 26:5310–5318
Shahbazian MD, Grunstein M (2007) Functions of site-specific histone acetylation and deacetylation. Annu Rev Biochem 76:75–100
Mellert HS, McMahon SB (2009) Biochemical pathways that regulate acetyltransferase and deacetylase activity in mammalian cells. Trends Biochem Sci 34:571–578
Tamaru H (2010) Confining euchromatin/heterochromatin territory: jumonji crosses the line. Genes Dev 24:1465–1478
Hodawadekar SC, Marmorstein R (2007) Chemistry of acetyl transfer by histone modifying enzymes: structure, mechanism and implications for effector design. Oncogene 26:5528–5540
Lee KK, Workman JL (2007) Histone acetyltransferase complexes: one size doesn’t fit all. Nat Rev Mol Cell Biol 8:284–295
Baker SP, Grant PA (2007) The SAGA continues: expanding the cellular role of a transcriptional co-activator complex. Oncogene 26:5329–5340
Nagy Z, Tora L (2007) Distinct GCN5/PCAF-containing complexes function as co-activators and are involved in transcription factor and global histone acetylation. Oncogene 26:5341–5357
Gray SG, Ekstrom TJ (2001) The human histone deacetylase family. Exp Cell Res 262:75–83
Fischle W, Dequiedt F, Fillion M, Hendzel MJ, Voelter W et al (2001) Human HDAC7 histone deacetylase activity is associated with HDAC3 in vivo. J Biol Chem 276:35826–35835
Fischle W, Dequiedt F, Hendzel MJ, Guenther MG, Lazar MA et al (2002) Enzymatic activity associated with class II HDACs is dependent on a multiprotein complex containing HDAC3 and SMRT/N-CoR. Mol Cell 9:45–57
Haigis MC, Guarente LP (2006) Mammalian sirtuins—emerging roles in physiology, aging, and calorie restriction. Genes Dev 20:2913–2921
Sauve AA, Wolberger C, Schramm VL, Boeke JD (2006) The biochemistry of sirtuins. Annu Rev Biochem 75:435–465
Gao L, Cueto MA, Asselbergs F, Atadja P (2002) Cloning and functional characterization of HDAC11, a novel member of the human histone deacetylase family. J Biol Chem 277:25748–25755
Barski A, Cuddapah S, Cui K, Roh TY, Schones DE et al (2007) High-resolution profiling of histone methylations in the human genome. Cell 129:823–837
Bedford MT, Clarke SG (2009) Protein arginine methylation in mammals: who, what, and why. Mol Cell 33:1–13
Lan F, Shi Y (2009) Epigenetic regulation: methylation of histone and non-histone proteins. Sci China C Life Sci 52:311–322
Ng SS, Yue WW, Oppermann U, Klose RJ (2009) Dynamic protein methylation in chromatin biology. Cell Mol Life Sci 66:407–422
Heintzman ND, Hon GC, Hawkins RD, Kheradpour P, Stark A et al (2009) Histone modifications at human enhancers reflect global cell-type-specific gene expression. Nature 459:108–112
Cheng X, Collins RE, Zhang X (2005) Structural and sequence motifs of protein (histone) methylation enzymes. Annu Rev Biophys Biomol Struct 34:267–294
Wolf SS (2009) The protein arginine methyltransferase family: an update about function, new perspectives and the physiological role in humans. Cell Mol Life Sci 66:2109–2121
Mosammaparast N, Shi Y (2010) Reversal of histone methylation: biochemical and molecular mechanisms of histone demethylases. Annu Rev Biochem 79:155–179
Shi Y, Lan F, Matson C, Mulligan P, Whetstine JR et al (2004) Histone demethylation mediated by the nuclear amine oxidase homolog LSD1. Cell 119:941–953
Chang B, Chen Y, Zhao Y, Bruick RK (2007) JMJD6 is a histone arginine demethylase. Science 318:444–447
Tsukada Y, Fang J, Erdjument-Bromage H, Warren ME, Borchers CH et al (2006) Histone demethylation by a family of JmjC domain-containing proteins. Nature 439:811–816
Whetstine JR, Nottke A, Lan F, Huarte M, Smolikov S et al (2006) Reversal of histone lysine trimethylation by the JMJD2 family of histone demethylases. Cell 125:467–481
Oki M, Aihara H, Ito T (2007) Role of histone phosphorylation in chromatin dynamics and its implications in diseases. Subcell Biochem 41:319–336
Dawson MA, Bannister AJ, Gottgens B, Foster SD, Bartke T et al (2009) JAK2 phosphorylates histone H3Y41 and excludes HP1alpha from chromatin. Nature 461:819–822
Baek SH (2011) When signaling kinases meet histones and histone modifiers in the nucleus. Mol Cell 42:274–284
Hu S, Xie Z, Onishi A, Yu X, Jiang L et al (2009) Profiling the human protein-DNA interactome reveals ERK2 as a transcriptional repressor of interferon signaling. Cell 139:610–622
Goto H, Yasui Y, Nigg EA, Inagaki M (2002) Aurora-B phosphorylates Histone H3 at serine28 with regard to the mitotic chromosome condensation. Genes Cells 7:11–17
Sugiyama K, Sugiura K, Hara T, Sugimoto K, Shima H et al (2002) Aurora-B associated protein phosphatases as negative regulators of kinase activation. Oncogene 21:3103–3111
Flaus A, Martin DM, Barton GJ, Owen-Hughes T (2006) Identification of multiple distinct Snf2 subfamilies with conserved structural motifs. Nucleic Acids Res 34:2887–2905
Bao Y, Shen X (2007) SnapShot: chromatin remodeling complexes. Cell 129:632
Wang GG, Allis CD, Chi P (2007) Chromatin remodeling and cancer, part II: ATP-dependent chromatin remodeling. Trends Mol Med 13:373–380
Clapier CR, Cairns BR (2009) The biology of chromatin remodeling complexes. Annu Rev Biochem 78:273–304
Lange M, Demajo S, Jain P, Di Croce L (2011) Combinatorial assembly and function of chromatin regulatory complexes. Epigenomics 3:567–580
Hargreaves DC, Crabtree GR (2011) ATP-dependent chromatin remodeling: genetics, genomics and mechanisms. Cell Res 21:396–420
Amaral PP, Dinger ME, Mercer TR, Mattick JS (2008) The eukaryotic genome as an RNA machine. Science 319:1787–1789
Guttman M, Amit I, Garber M, French C, Lin MF et al (2009) Chromatin signature reveals over a thousand highly conserved large non-coding RNAs in mammals. Nature 458:223–227
Guttman M, Garber M, Levin JZ, Donaghey J, Robinson J et al (2010) Ab initio reconstruction of cell type-specific transcriptomes in mouse reveals the conserved multi-exonic structure of lincRNAs. Nat Biotechnol 28:503–510
Alexander RP, Fang G, Rozowsky J, Snyder M, Gerstein MB (2010) Annotating non-coding regions of the genome. Nat Rev Genet 11:559–571
Taft RJ, Pang KC, Mercer TR, Dinger M, Mattick JS (2010) Non-coding RNAs: regulators of disease. J Pathol 220:126–139
Esteller M (2011) Non-coding RNAs in human disease. Nat Rev Genet 12:861–874
Kaikkonen MU, Lam MT, Glass CK (2011) Non-coding RNAs as regulators of gene expression and epigenetics. Cardiovasc Res 90:430–440
Mercer TR, Dinger ME, Mattick JS (2009) Long non-coding RNAs: insights into functions. Nat Rev Genet 10:155–159
Small EM, Olson EN (2011) Pervasive roles of microRNAs in cardiovascular biology. Nature 469:336–342
Mendell JT, Olson EN (2012) MicroRNAs in stress signaling and human disease. Cell 148:1172–1187
Wapinski O, Chang HY (2011) Long noncoding RNAs and human disease. Trends Cell Biol 21:354–361
Carthew RW, Sontheimer EJ (2009) Origins and mechanisms of miRNAs and siRNAs. Cell 136:642–655
Malone CD, Hannon GJ (2009) Small RNAs as guardians of the genome. Cell 136:656–668
Ghildiyal M, Zamore PD (2009) Small silencing RNAs: an expanding universe. Nat Rev Genet 10:94–108
Bartel DP (2004) MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116:281–297
Liu N, Olson EN (2010) MicroRNA regulatory networks in cardiovascular development. Dev Cell 18:510–525
van Rooij E (2011) The art of microRNA research. Circ Res 108:219–234
Kapranov P, Cheng J, Dike S, Nix DA, Duttagupta R et al (2007) RNA maps reveal new RNA classes and a possible function for pervasive transcription. Science 316:1484–1488
Nakaya HI, Amaral PP, Louro R, Lopes A, Fachel AA et al (2007) Genome mapping and expression analyses of human intronic noncoding RNAs reveal tissue-specific patterns and enrichment in genes related to regulation of transcription. Genome Biol 8:R43
Cabili MN, Trapnell C, Goff L, Koziol M, Tazon-Vega B et al (2011) Integrative annotation of human large intergenic noncoding RNAs reveals global properties and specific subclasses. Genes Dev 25:1915–1927
Derrien T, Johnson R, Bussotti G, Tanzer A, Djebali S et al (2012) The GENCODE v7 catalog of human long noncoding RNAs: analysis of their gene structure, evolution, and expression. Genome Res 22:1775–1789
Nagano T, Fraser P (2011) No-nonsense functions for long noncoding RNAs. Cell 145:178–181
Wang KC, Chang HY (2011) Molecular mechanisms of long noncoding RNAs. Mol Cell 43:904–914
Guttman M, Rinn JL (2012) Modular regulatory principles of large non-coding RNAs. Nature 482:339–346
Magistri M, Faghihi MA, St Laurent G, Wahlestedt C III (2012) Regulation of chromatin structure by long noncoding RNAs: focus on natural antisense transcripts. Trends Genet 28:389–396
Rinn JL, Chang HY (2012) Genome regulation by long noncoding RNAs. Annu Rev Biochem 81:145–166
Geisler S, Coller J (2013) RNA in unexpected places: long non-coding RNA functions in diverse cellular contexts. Nat Rev Mol Cell Biol 14:699–712
Mercer TR, Mattick JS (2013) Structure and function of long noncoding RNAs in epigenetic regulation. Nat Struct Mol Biol 20:300–307
Sabatti C, Service SK, Hartikainen AL, Pouta A, Ripatti S et al (2009) Genome-wide association analysis of metabolic traits in a birth cohort from a founder population. Nat Genet 41:35–46
Marian AJ, Belmont J (2011) Strategic approaches to unraveling genetic causes of cardiovascular diseases. Circ Res 108:1252–1269
Kathiresan S, Srivastava D (2012) Genetics of human cardiovascular disease. Cell 148:1242–1257
Esteller M (2008) Epigenetics in cancer. N Engl J Med 358:1148–1159
Baylin SB, Jones PA (2011) A decade of exploring the cancer epigenome—biological and translational implications. Nat Rev Cancer 11:726–734
Iorio MV, Croce CM (2012) MicroRNA dysregulation in cancer: diagnostics, monitoring and therapeutics. A comprehensive review. EMBO Mol Med 4:143–159
Malumbres M (2013) miRNAs and cancer: an epigenetics view. Mol Aspects Med 34:863–874
Ling C, Groop L (2009) Epigenetics: a molecular link between environmental factors and type 2 diabetes. Diabetes 58:2718–2725
MacFarlane AJ, Strom A, Scott FW (2009) Epigenetics: deciphering how environmental factors may modify autoimmune type 1 diabetes. Mamm Genome 20:624–632
Mill J, Tang T, Kaminsky Z, Khare T, Yazdanpanah S et al (2008) Epigenomic profiling reveals DNA-methylation changes associated with major psychosis. Am J Hum Genet 82:696–711
Singh SM, O’Reilly R (2009) (Epi)genomics and neurodevelopment in schizophrenia: monozygotic twins discordant for schizophrenia augment the search for disease-related (epi)genomic alterations. Genome 52:8–19
Zaina S, Lund G (2013) Atherosclerosis: cell biology and lipoproteins–panoramic views of DNA methylation landscapes of atherosclerosis. Curr Opin Lipidol 24:369–370
Zaina S (2014) Unraveling the DNA methylome of atherosclerosis. Curr Opin Lipidol 25:148–153
Sharma P, Kumar J, Garg G, Kumar A, Patowary A et al (2008) Detection of altered global DNA methylation in coronary artery disease patients. DNA Cell Biol 27:357–365
Hiltunen MO, Turunen MP, Hakkinen TP, Rutanen J, Hedman M et al (2002) DNA hypomethylation and methyltransferase expression in atherosclerotic lesions. Vasc Med 7:5–11
Duthie SJ (2011) Epigenetic modifications and human pathologies: cancer and CVD. Proc Nutr Soc 70:47–56
Lund G, Andersson L, Lauria M, Lindholm M, Fraga MF et al (2004) DNA methylation polymorphisms precede any histological sign of atherosclerosis in mice lacking apolipoprotein E. J Biol Chem 279:29147–29154
Nazarenko MS, Puzyrev VP, Lebedev IN, Frolov AV, Barbarash OL et al (2011) Methylation profiling of human atherosclerotic plaques. Mol Biol (Mosk) 45:610–616
Baccarelli A, Wright R, Bollati V, Litonjua A, Zanobetti A et al (2010) Ischemic heart disease and stroke in relation to blood DNA methylation. Epidemiology 21:819–828
Kim M, Long TI, Arakawa K, Wang R, Yu MC et al (2010) DNA methylation as a biomarker for cardiovascular disease risk. PLoS One 5:e9692
Post WS, Goldschmidt-Clermont PJ, Wilhide CC, Heldman AW, Sussman MS et al (1999) Methylation of the estrogen receptor gene is associated with aging and atherosclerosis in the cardiovascular system. Cardiovasc Res 43:985–991
Ying AK, Hassanain HH, Roos CM, Smiraglia DJ, Issa JJ et al (2000) Methylation of the estrogen receptor-alpha gene promoter is selectively increased in proliferating human aortic smooth muscle cells. Cardiovasc Res 46:172–179
Kim J, Kim JY, Song KS, Lee YH, Seo JS et al (2007) Epigenetic changes in estrogen receptor beta gene in atherosclerotic cardiovascular tissues and in vitro vascular senescence. Biochim Biophys Acta 1772:72–80
Jia L, Zhu L, Wang JZ, Wang XJ, Chen JZ et al (2013) Methylation of FOXP3 in regulatory T cells is related to the severity of coronary artery disease. Atherosclerosis 228:346–352
Lu CX, Xu RD, Cao M, Wang G, Yan FQ et al (2013) FOXP3 demethylation as a means of identifying quantitative defects in regulatory T cells in acute coronary syndrome. Atherosclerosis 229:263–270
Chan Y, Fish JE, D’Abreo C, Lin S, Robb GB et al (2004) The cell-specific expression of endothelial nitric-oxide synthase: a role for DNA methylation. J Biol Chem 279:35087–35100
Girelli D, Russo C, Ferraresi P, Olivieri O, Pinotti M et al (2000) Polymorphisms in the factor VII gene and the risk of myocardial infarction in patients with coronary artery disease. N Engl J Med 343:774–780
Friso S, Lotto V, Choi SW, Girelli D, Pinotti M et al (2012) Promoter methylation in coagulation F7 gene influences plasma FVII concentrations and relates to coronary artery disease. J Med Genet 49:192–199
Riviere G, Lienhard D, Andrieu T, Vieau D, Frey BM et al (2011) Epigenetic regulation of somatic angiotensin-converting enzyme by DNA methylation and histone acetylation. Epigenetics 6:478–489
Friso S, Pizzolo F, Choi SW, Guarini P, Castagna A et al (2008) Epigenetic control of 11 beta-hydroxysteroid dehydrogenase 2 gene promoter is related to human hypertension. Atherosclerosis 199:323–327
Lee HA, Baek I, Seok YM, Yang E, Cho HM et al (2010) Promoter hypomethylation upregulates Na+–K+–2Cl– cotransporter 1 in spontaneously hypertensive rats. Biochem Biophys Res Commun 396:252–257
Cho HM, Lee HA, Kim HY, Han HS, Kim IK (2011) Expression of Na+–K+–2Cl– cotransporter 1 is epigenetically regulated during postnatal development of hypertension. Am J Hypertens 24:1286–1293
Fatima N, Schooley JF Jr, Claycomb WC, Flagg TP (2012) Promoter DNA methylation regulates murine SUR1 (Abcc8) and SUR2 (Abcc9) expression in HL-1 cardiomyocytes. PLoS One 7:e41533
Movassagh M, Choy MK, Goddard M, Bennett MR, Down TA et al (2010) Differential DNA methylation correlates with differential expression of angiogenic factors in human heart failure. PLoS One 5:e8564
Movassagh M, Choy MK, Knowles DA, Cordeddu L, Haider S et al (2011) Distinct epigenomic features in end-stage failing human hearts. Circulation 124:2411–2422
Lu Z, Scott I, Webster BR, Sack MN (2009) The emerging characterization of lysine residue deacetylation on the modulation of mitochondrial function and cardiovascular biology. Circ Res 105:830–841
Haas J, Frese KS, Park YJ, Keller A, Vogel B et al (2013) Alterations in cardiac DNA methylation in human dilated cardiomyopathy. EMBO Mol Med 5:413–429
Meurs KM, Mealey KL (2008) Evaluation of the flanking nucleotide sequences of sarcomeric hypertrophic cardiomyopathy substitution mutations. Mutat Res 642:86–89
Meurs KM, Kuan M (2011) Differential methylation of CpG sites in two isoforms of myosin binding protein C, an important hypertrophic cardiomyopathy gene. Environ Mol Mutagen 52:161–164
Kao YH, Chen YC, Cheng CC, Lee TI, Chen YJ et al (2010) Tumor necrosis factor-alpha decreases sarcoplasmic reticulum Ca2+-ATPase expressions via the promoter methylation in cardiomyocytes. Crit Care Med 38:217–222
Kao YH, Cheng CC, Chen YC, Chung CC, Lee TI et al (2011) Hydralazine-induced promoter demethylation enhances sarcoplasmic reticulum Ca2+-ATPase and calcium homeostasis in cardiac myocytes. Lab Invest 91:1291–1297
Kao YH, Chen YC, Chung CC, Lien GS, Chen SA et al (2013) Heart failure and angiotensin II modulate atrial Pitx2c promotor methylation. Clin Exp Pharmacol Physiol 40:379–384
Zarain-Herzberg A, Afzal N, Elimban V, Dhalla NS (1996) Decreased expression of cardiac sarcoplasmic reticulum Ca(2+)-pump ATPase in congestive heart failure due to myocardial infarction. Mol Cell Biochem 163–164:285–290
Kranias EG, Hajjar RJ (2012) Modulation of cardiac contractility by the phospholamban/SERCA2a regulatome. Circ Res 110:1646–1660
Maisel WH, Stevenson LW (2003) Atrial fibrillation in heart failure: epidemiology, pathophysiology, and rationale for therapy. Am J Cardiol 91:2D–8D
Kirchhof P, Kahr PC, Kaese S, Piccini I, Vokshi I et al (2011) PITX2c is expressed in the adult left atrium, and reducing Pitx2c expression promotes atrial fibrillation inducibility and complex changes in gene expression. Circ Cardiovasc Genet 4:123–133
Chinchilla A, Daimi H, Lozano-Velasco E, Dominguez JN, Caballero R et al (2011) PITX2 insufficiency leads to atrial electrical and structural remodeling linked to arrhythmogenesis. Circ Cardiovasc Genet 4:269–279
Doyama K, Fujiwara H, Fukumoto M, Tanaka M, Fujiwara Y et al (1996) Tumour necrosis factor is expressed in cardiac tissues of patients with heart failure. Int J Cardiol 54:217–225
Habib FM, Springall DR, Davies GJ, Oakley CM, Yacoub MH et al (1996) Tumour necrosis factor and inducible nitric oxide synthase in dilated cardiomyopathy. Lancet 347:1151–1155
Torre-Amione G, Kapadia S, Lee J, Durand JB, Bies RD et al (1996) Tumor necrosis factor-alpha and tumor necrosis factor receptors in the failing human heart. Circulation 93:704–711
Unger T, Li J (2004) The role of the renin–angiotensin–aldosterone system in heart failure. J Renin Angiotensin Aldosterone Syst 5(Suppl 1):S7–S10
Kox M, Pickkers P (2010) Poison to the heart. Crit Care Med 38:331–332
Kao YH, Lien GS, Chao TF, Chen YJ (2014) DNA methylation inhibition: a novel therapeutic strategy for heart failure. Int J Cardiol 176:232–233
Yao TP, Oh SP, Fuchs M, Zhou ND, Ch’ng LE et al (1998) Gene dosage-dependent embryonic development and proliferation defects in mice lacking the transcriptional integrator p300. Cell 93:361–372
Shikama N, Lutz W, Kretzschmar R, Sauter N, Roth JF et al (2003) Essential function of p300 acetyltransferase activity in heart, lung and small intestine formation. EMBO J 22:5175–5185
Gusterson RJ, Jazrawi E, Adcock IM, Latchman DS (2003) The transcriptional co-activators CREB-binding protein (CBP) and p300 play a critical role in cardiac hypertrophy that is dependent on their histone acetyltransferase activity. J Biol Chem 278:6838–6847
Morita H, Nagai R, Seidman JG, Seidman CE (2010) Sarcomere gene mutations in hypertrophy and heart failure. J Cardiovasc Transl Res 3:297–303
Miyamoto S, Kawamura T, Morimoto T, Ono K, Wada H et al (2006) Histone acetyltransferase activity of p300 is required for the promotion of left ventricular remodeling after myocardial infarction in adult mice in vivo. Circulation 113:679–690
Yanazume T, Hasegawa K, Morimoto T, Kawamura T, Wada H et al (2003) Cardiac p300 is involved in myocyte growth with decompensated heart failure. Mol Cell Biol 23:3593–3606
Slepak TI, Webster KA, Zang J, Prentice H, O’Dowd A et al (2001) Control of cardiac-specific transcription by p300 through myocyte enhancer factor-2D. J Biol Chem 276:7575–7585
Ma K, Chan JK, Zhu G, Wu Z (2005) Myocyte enhancer factor 2 acetylation by p300 enhances its DNA binding activity, transcriptional activity, and myogenic differentiation. Mol Cell Biol 25:3575–3582
Wei JQ, Shehadeh LA, Mitrani JM, Pessanha M, Slepak TI et al (2008) Quantitative control of adaptive cardiac hypertrophy by acetyltransferase p300. Circulation 118:934–946
Hennekam RC (2006) Rubinstein–Taybi syndrome. Eur J Hum Genet 14:981–985
Thienpont B, Breckpot J, Holvoet M, Vermeesch JR, Devriendt K (2007) A microduplication of CBP in a patient with mental retardation and a congenital heart defect. Am J Med Genet A 143A:2160–2164
Thienpont B, Bena F, Breckpot J, Philip N, Menten B et al (2010) Duplications of the critical Rubinstein–Taybi deletion region on chromosome 16p13.3 cause a novel recognisable syndrome. J Med Genet 47:155–161
Han P, Hang CT, Yang J, Chang CP (2011) Chromatin remodeling in cardiovascular development and physiology. Circ Res 108:378–396
Ohtani K, Dimmeler S (2011) Epigenetic regulation of cardiovascular differentiation. Cardiovasc Res 90:404–412
Tingare A, Thienpont B, Roderick HL (2013) Epigenetics in the heart: the role of histone modifications in cardiac remodelling. Biochem Soc Trans 41:789–796
Montgomery RL, Davis CA, Potthoff MJ, Haberland M, Fielitz J et al (2007) Histone deacetylases 1 and 2 redundantly regulate cardiac morphogenesis, growth, and contractility. Genes Dev 21:1790–1802
Cao DJ, Wang ZV, Battiprolu PK, Jiang N, Morales CR et al (2011) Histone deacetylase (HDAC) inhibitors attenuate cardiac hypertrophy by suppressing autophagy. Proc Natl Acad Sci USA 108:4123–4128
Montgomery RL, Potthoff MJ, Haberland M, Qi X, Matsuzaki S et al (2008) Maintenance of cardiac energy metabolism by histone deacetylase 3 in mice. J Clin Invest 118:3588–3597
Trivedi CM, Lu MM, Wang Q, Epstein JA (2008) Transgenic overexpression of Hdac3 in the heart produces increased postnatal cardiac myocyte proliferation but does not induce hypertrophy. J Biol Chem 283:26484–26489
Zhang CL, McKinsey TA, Chang S, Antos CL, Hill JA et al (2002) Class II histone deacetylases act as signal-responsive repressors of cardiac hypertrophy. Cell 110:479–488
Chang S, McKinsey TA, Zhang CL, Richardson JA, Hill JA et al (2004) Histone deacetylases 5 and 9 govern responsiveness of the heart to a subset of stress signals and play redundant roles in heart development. Mol Cell Biol 24:8467–8476
Chang S, Young BD, Li S, Qi X, Richardson JA et al (2006) Histone deacetylase 7 maintains vascular integrity by repressing matrix metalloproteinase 10. Cell 126:321–334
Vega RB, Matsuda K, Oh J, Barbosa AC, Yang X et al (2004) Histone deacetylase 4 controls chondrocyte hypertrophy during skeletogenesis. Cell 119:555–566
Zhang Y, Kwon S, Yamaguchi T, Cubizolles F, Rousseaux S et al (2008) Mice lacking histone deacetylase 6 have hyperacetylated tubulin but are viable and develop normally. Mol Cell Biol 28:1688–1701
Williams SR, Aldred MA, Der Kaloustian VM, Halal F, Gowans G et al (2010) Haploinsufficiency of HDAC4 causes brachydactyly mental retardation syndrome, with brachydactyly type E, developmental delays, and behavioral problems. Am J Hum Genet 87:219–228
Zhang D, Wu CT, Qi X, Meijering RA, Hoogstra-Berends F et al (2014) Activation of histone deacetylase-6 induces contractile dysfunction through derailment of alpha-tubulin proteostasis in experimental and human atrial fibrillation. Circulation 129:346–358
Cheng HL, Mostoslavsky R, Saito S, Manis JP, Gu Y et al (2003) Developmental defects and p53 hyperacetylation in Sir2 homolog (SIRT1)-deficient mice. Proc Natl Acad Sci USA 100:10794–10799
Bordone L, Cohen D, Robinson A, Motta MC, van Veen E et al (2007) SIRT1 transgenic mice show phenotypes resembling calorie restriction. Aging Cell 6:759–767
Zhou B, Wu LJ, Li LH, Tashiro S, Onodera S et al (2006) Silibinin protects against isoproterenol-induced rat cardiac myocyte injury through mitochondrial pathway after up-regulation of SIRT1. J Pharmacol Sci 102:387–395
Outeiro TF, Kontopoulos E, Altmann SM, Kufareva I, Strathearn KE et al (2007) Sirtuin 2 inhibitors rescue alpha-synuclein-mediated toxicity in models of Parkinson’s disease. Science 317:516–519
Lynn EG, McLeod CJ, Gordon JP, Bao J, Sack MN (2008) SIRT2 is a negative regulator of anoxia-reoxygenation tolerance via regulation of 14-3-3 zeta and BAD in H9c2 cells. FEBS Lett 582:2857–2862
Lombard DB, Alt FW, Cheng HL, Bunkenborg J, Streeper RS et al (2007) Mammalian Sir2 homolog SIRT3 regulates global mitochondrial lysine acetylation. Mol Cell Biol 27:8807–8814
Sundaresan NR, Gupta M, Kim G, Rajamohan SB, Isbatan A et al (2009) Sirt3 blocks the cardiac hypertrophic response by augmenting Foxo3a-dependent antioxidant defense mechanisms in mice. J Clin Invest 119:2758–2771
Sundaresan NR, Samant SA, Pillai VB, Rajamohan SB, Gupta MP (2008) SIRT3 is a stress-responsive deacetylase in cardiomyocytes that protects cells from stress-mediated cell death by deacetylation of Ku70. Mol Cell Biol 28:6384–6401
Vakhrusheva O, Smolka C, Gajawada P, Kostin S, Boettger T et al (2008) Sirt7 increases stress resistance of cardiomyocytes and prevents apoptosis and inflammatory cardiomyopathy in mice. Circ Res 102:703–710
Mostoslavsky R, Chua KF, Lombard DB, Pang WW, Fischer MR et al (2006) Genomic instability and aging-like phenotype in the absence of mammalian SIRT6. Cell 124:315–329
Sundaresan NR, Vasudevan P, Zhong L, Kim G, Samant S et al (2012) The sirtuin SIRT6 blocks IGF-Akt signaling and development of cardiac hypertrophy by targeting c-Jun. Nat Med 18:1643–1650
Ruthenburg AJ, Allis CD, Wysocka J (2007) Methylation of lysine 4 on histone H3: intricacy of writing and reading a single epigenetic mark. Mol Cell 25:15–30
Kaneda R, Takada S, Yamashita Y, Choi YL, Nonaka-Sarukawa M et al (2009) Genome-wide histone methylation profile for heart failure. Genes Cells 14:69–77
Stein AB, Jones TA, Herron TJ, Patel SR, Day SM et al (2011) Loss of H3K4 methylation destabilizes gene expression patterns and physiological functions in adult murine cardiomyocytes. J Clin Invest 121:2641–2650
Radicke S, Cotella D, Graf EM, Banse U, Jost N et al (2006) Functional modulation of the transient outward current Ito by KCNE beta-subunits and regional distribution in human non-failing and failing hearts. Cardiovasc Res 71:695–703
Pandya K, Kohro T, Mimura I, Kobayashi M, Wada Y et al (2012) Distribution of histone3 lysine 4 trimethylation at T3-responsive loci in the heart during reversible changes in gene expression. Gene Expr 15:183–198
Zhang QJ, Chen HZ, Wang L, Liu DP, Hill JA et al (2011) The histone trimethyllysine demethylase JMJD2A promotes cardiac hypertrophy in response to hypertrophic stimuli in mice. J Clin Invest 121:2447–2456
Sheikh F, Raskin A, Chu PH, Lange S, Domenighetti AA et al (2008) An FHL1-containing complex within the cardiomyocyte sarcomere mediates hypertrophic biomechanical stress responses in mice. J Clin Invest 118:3870–3880
Epsztejn-Litman S, Feldman N, Abu-Remaileh M, Shufaro Y, Gerson A et al (2008) De novo DNA methylation promoted by G9a prevents reprogramming of embryonically silenced genes. Nat Struct Mol Biol 15:1176–1183
Tachibana M, Matsumura Y, Fukuda M, Kimura H, Shinkai Y (2008) G9a/GLP complexes independently mediate H3K9 and DNA methylation to silence transcription. EMBO J 27:2681–2690
Dong KB, Maksakova IA, Mohn F, Leung D, Appanah R et al (2008) DNA methylation in ES cells requires the lysine methyltransferase G9a but not its catalytic activity. EMBO J 27:2691–2701
Feng Q, Wang H, Ng HH, Erdjument-Bromage H, Tempst P et al (2002) Methylation of H3-lysine 79 is mediated by a new family of HMTases without a SET domain. Curr Biol 12:1052–1058
Ng HH, Feng Q, Wang H, Erdjument-Bromage H, Tempst P et al (2002) Lysine methylation within the globular domain of histone H3 by Dot1 is important for telomeric silencing and Sir protein association. Genes Dev 16:1518–1527
Schubeler D, MacAlpine DM, Scalzo D, Wirbelauer C, Kooperberg C et al (2004) The histone modification pattern of active genes revealed through genome-wide chromatin analysis of a higher eukaryote. Genes Dev 18:1263–1271
Steger DJ, Lefterova MI, Ying L, Stonestrom AJ, Schupp M et al (2008) DOT1L/KMT4 recruitment and H3K79 methylation are ubiquitously coupled with gene transcription in mammalian cells. Mol Cell Biol 28:2825–2839
Jones B, Su H, Bhat A, Lei H, Bajko J et al (2008) The histone H3K79 methyltransferase Dot1L is essential for mammalian development and heterochromatin structure. PLoS Genet 4:e1000190
Nguyen AT, Xiao B, Neppl RL, Kallin EM, Li J et al (2011) DOT1L regulates dystrophin expression and is critical for cardiac function. Genes Dev 25:263–274
Seidman JG, Seidman C (2001) The genetic basis for cardiomyopathy: from mutation identification to mechanistic paradigms. Cell 104:557–567
Zhang Y, Reinberg D (2001) Transcription regulation by histone methylation: interplay between different covalent modifications of the core histone tails. Genes Dev 15:2343–2360
Rada-Iglesias A, Bajpai R, Swigut T, Brugmann SA, Flynn RA et al (2011) A unique chromatin signature uncovers early developmental enhancers in humans. Nature 470:279–283
Hohl M, Wagner M, Reil JC, Muller SA, Tauchnitz M et al (2013) HDAC4 controls histone methylation in response to elevated cardiac load. J Clin Invest 123:1359–1370
Mathiyalagan P, Okabe J, Chang L, Su Y, Du XJ et al (2014) The primary microRNA-208b interacts with Polycomb-group protein, Ezh2, to regulate gene expression in the heart. Nucleic Acids Res 42:790–803
Bevilacqua A, Willis MS, Bultman SJ (2014) SWI/SNF chromatin-remodeling complexes in cardiovascular development and disease. Cardiovasc Pathol 23:85–91
Takeuchi JK, Lou X, Alexander JM, Sugizaki H, Delgado-Olguin P et al (2011) Chromatin remodelling complex dosage modulates transcription factor function in heart development. Nat Commun 2:187
Stankunas K, Hang CT, Tsun ZY, Chen H, Lee NV et al (2008) Endocardial Brg1 represses ADAMTS1 to maintain the microenvironment for myocardial morphogenesis. Dev Cell 14:298–311
Jenni R, Rojas J, Oechslin E (1999) Isolated noncompaction of the myocardium. N Engl J Med 340:966–967
Hang CT, Yang J, Han P, Cheng HL, Shang C et al (2010) Chromatin regulation by Brg1 underlies heart muscle development and disease. Nature 466:62–67
Lickert H, Takeuchi JK, Von Both I, Walls JR, McAuliffe F et al (2004) Baf60c is essential for function of BAF chromatin remodelling complexes in heart development. Nature 432:107–112
Kadoch C, Hargreaves DC, Hodges C, Elias L, Ho L et al (2013) Proteomic and bioinformatic analysis of mammalian SWI/SNF complexes identifies extensive roles in human malignancy. Nat Genet 45:592–601
Mehrotra A, Joe B, de la Serna IL (2013) SWI/SNF chromatin remodeling enzymes are associated with cardiac hypertrophy in a genetic rat model of hypertension. J Cell Physiol 228:2337–2342
Wang Z, Zhai W, Richardson JA, Olson EN, Meneses JJ et al (2004) Polybromo protein BAF180 functions in mammalian cardiac chamber maturation. Genes Dev 18:3106–3116
Nagl NG Jr, Wang X, Patsialou A, Van Scoy M, Moran E (2007) Distinct mammalian SWI/SNF chromatin remodeling complexes with opposing roles in cell-cycle control. EMBO J 26:752–763
Chandler RL, Brennan J, Schisler JC, Serber D, Patterson C et al (2013) ARID1a-DNA interactions are required for promoter occupancy by SWI/SNF. Mol Cell Biol 33:265–280
Lei I, Gao X, Sham MH, Wang Z (2012) SWI/SNF protein component BAF250a regulates cardiac progenitor cell differentiation by modulating chromatin accessibility during second heart field development. J Biol Chem 287:24255–24262
Tsurusaki Y, Okamoto N, Ohashi H, Kosho T, Imai Y et al (2012) Mutations affecting components of the SWI/SNF complex cause Coffin-Siris syndrome. Nat Genet 44:376–378
Santen GW, Aten E, Sun Y, Almomani R, Gilissen C et al (2012) Mutations in SWI/SNF chromatin remodeling complex gene ARID1B cause Coffin-Siris syndrome. Nat Genet 44:379–380
Van Houdt JK, Nowakowska BA, Sousa SB, van Schaik BD, Seuntjens E et al (2012) Heterozygous missense mutations in SMARCA2 cause Nicolaides–Baraitser syndrome. Nat Genet 44(445–449):S441
Kosho T, Okamoto N, Ohashi H, Tsurusaki Y, Imai Y et al (2013) Clinical correlations of mutations affecting six components of the SWI/SNF complex: detailed description of 21 patients and a review of the literature. Am J Med Genet A 161A:1221–1237
Vissers LE, van Ravenswaaij CM, Admiraal R, Hurst JA, de Vries BB et al (2004) Mutations in a new member of the chromodomain gene family cause CHARGE syndrome. Nat Genet 36:955–957
Bajpai R, Chen DA, Rada-Iglesias A, Zhang J, Xiong Y et al (2010) CHD7 cooperates with PBAF to control multipotent neural crest formation. Nature 463:958–962
Li W, Xiong Y, Shang C, Twu KY, Hang CT et al (2013) Brg1 governs distinct pathways to direct multiple aspects of mammalian neural crest cell development. Proc Natl Acad Sci USA 110:1738–1743
Chen J, Wang DZ (2012) microRNAs in cardiovascular development. J Mol Cell Cardiol 52:949–957
Zampetaki A, Mayr M (2012) MicroRNAs in vascular and metabolic disease. Circ Res 110:508–522
Quiat D, Olson EN (2013) MicroRNAs in cardiovascular disease: from pathogenesis to prevention and treatment. J Clin Invest 123:11–18
Kumarswamy R, Thum T (2013) Non-coding RNAs in cardiac remodeling and heart failure. Circ Res 113:676–689
Tsai MC, Spitale RC, Chang HY (2011) Long intergenic noncoding RNAs: new links in cancer progression. Cancer Res 71:3–7
Gutschner T, Diederichs S (2012) The hallmarks of cancer: a long non-coding RNA point of view. RNA Biol 9:703–719
Kim T, Reitmair A (2013) Non-coding RNAs: functional aspects and diagnostic utility in oncology. Int J Mol Sci 14:4934–4968
Li D, Chen G, Yang J, Fan X, Gong Y et al (2013) Transcriptome analysis reveals distinct patterns of long noncoding RNAs in heart and plasma of mice with heart failure. PLoS One 8:e77938
Ounzain S, Micheletti R, Beckmann T, Schroen B, Alexanian M et al (2015) Genome-wide profiling of the cardiac transcriptome after myocardial infarction identifies novel heart-specific long non-coding RNAs. Eur Heart J 36:353–368
Ounzain S, Pezzuto I, Micheletti R, Burdet F, Sheta R et al (2014) Functional importance of cardiac enhancer-associated noncoding RNAs in heart development and disease. J Mol Cell Cardiol 76:55–70
Cesana M, Cacchiarelli D, Legnini I, Santini T, Sthandier O et al (2011) A long noncoding RNA controls muscle differentiation by functioning as a competing endogenous RNA. Cell 147:358–369
Lin Q, Schwarz J, Bucana C, Olson EN (1997) Control of mouse cardiac morphogenesis and myogenesis by transcription factor MEF2C. Science 276:1404–1407
Wang K, Liu F, Zhou LY, Long B, Yuan SM et al (2014) The long noncoding RNA CHRF regulates cardiac hypertrophy by targeting miR-489. Circ Res 114:1377–1388
Klattenhoff CA, Scheuermann JC, Surface LE, Bradley RK, Fields PA et al (2013) Braveheart, a long noncoding RNA required for cardiovascular lineage commitment. Cell 152:570–583
Chooniedass-Kothari S, Emberley E, Hamedani MK, Troup S, Wang X et al (2004) The steroid receptor RNA activator is the first functional RNA encoding a protein. FEBS Lett 566:43–47
Hube F, Guo J, Chooniedass-Kothari S, Cooper C, Hamedani MK et al (2006) Alternative splicing of the first intron of the steroid receptor RNA activator (SRA) participates in the generation of coding and noncoding RNA isoforms in breast cancer cell lines. DNA Cell Biol 25:418–428
Caretti G, Schiltz RL, Dilworth FJ, Di Padova M, Zhao P et al (2006) The RNA helicases p68/p72 and the noncoding RNA SRA are coregulators of MyoD and skeletal muscle differentiation. Dev Cell 11:547–560
Hube F, Velasco G, Rollin J, Furling D, Francastel C (2011) Steroid receptor RNA activator protein binds to and counteracts SRA RNA-mediated activation of MyoD and muscle differentiation. Nucleic Acids Res 39:513–525
Friedrichs F, Zugck C, Rauch GJ, Ivandic B, Weichenhan D et al (2009) HBEGF, SRA1, and IK: three cosegregating genes as determinants of cardiomyopathy. Genome Res 19:395–403
Grote P, Wittler L, Hendrix D, Koch F, Wahrisch S et al (2013) The tissue-specific lncRNA Fendrr is an essential regulator of heart and body wall development in the mouse. Dev Cell 24:206–214
Sauvageau M, Goff LA, Lodato S, Bonev B, Groff AF et al (2013) Multiple knockout mouse models reveal lincRNAs are required for life and brain development. Elife 2:e01749
Pandey RR, Mondal T, Mohammad F, Enroth S, Redrup L et al (2008) Kcnq1ot1 antisense noncoding RNA mediates lineage-specific transcriptional silencing through chromatin-level regulation. Mol Cell 32:232–246
Korostowski L, Sedlak N, Engel N (2012) The Kcnq1ot1 long non-coding RNA affects chromatin conformation and expression of Kcnq1, but does not regulate its imprinting in the developing heart. PLoS Genet 8:e1002956
Bokil NJ, Baisden JM, Radford DJ, Summers KM (2010) Molecular genetics of long QT syndrome. Mol Genet Metab 101:1–8
Ishii N, Ozaki K, Sato H, Mizuno H, Saito S et al (2006) Identification of a novel non-coding RNA, MIAT, that confers risk of myocardial infarction. J Hum Genet 51:1087–1099
Tsuiji H, Yoshimoto R, Hasegawa Y, Furuno M, Yoshida M et al (2011) Competition between a noncoding exon and introns: Gomafu contains tandem UACUAAC repeats and associates with splicing factor-1. Genes Cells 16:479–490
Lee JH, Gao C, Peng G, Greer C, Ren S et al (2011) Analysis of transcriptome complexity through RNA sequencing in normal and failing murine hearts. Circ Res 109:1332–1341
McPherson R, Pertsemlidis A, Kavaslar N, Stewart A, Roberts R et al (2007) A common allele on chromosome 9 associated with coronary heart disease. Science 316:1488–1491
Broadbent HM, Peden JF, Lorkowski S, Goel A, Ongen H et al (2008) Susceptibility to coronary artery disease and diabetes is encoded by distinct, tightly linked SNPs in the ANRIL locus on chromosome 9p. Hum Mol Genet 17:806–814
Congrains A, Kamide K, Ohishi M, Rakugi H (2013) ANRIL: molecular mechanisms and implications in human health. Int J Mol Sci 14:1278–1292
Yap KL, Li S, Munoz-Cabello AM, Raguz S, Zeng L et al (2010) Molecular interplay of the noncoding RNA ANRIL and methylated histone H3 lysine 27 by polycomb CBX7 in transcriptional silencing of INK4a. Mol Cell 38:662–674
Holdt LM, Beutner F, Scholz M, Gielen S, Gabel G et al (2010) ANRIL expression is associated with atherosclerosis risk at chromosome 9p21. Arterioscler Thromb Vasc Biol 30:620–627
Congrains A, Kamide K, Oguro R, Yasuda O, Miyata K et al (2012) Genetic variants at the 9p21 locus contribute to atherosclerosis through modulation of ANRIL and CDKN2A/B. Atherosclerosis 220:449–455
Kumarswamy R, Bauters C, Volkmann I, Maury F, Fetisch J et al (2014) Circulating long noncoding RNA, LIPCAR, predicts survival in patients with heart failure. Circ Res 114:1569–1575
Vausort M, Wagner DR, Devaux Y (2014) Long noncoding RNAs in patients with acute myocardial infarction. Circ Res 115:668–677
Yang KC, Yamada KA, Patel AY, Topkara VK, George I et al (2014) Deep RNA sequencing reveals dynamic regulation of myocardial noncoding RNAs in failing human heart and remodeling with mechanical circulatory support. Circulation 129:1009–1021
Han P, Li W, Lin CH, Yang J, Shang C et al (2014) A long noncoding RNA protects the heart from pathological hypertrophy. Nature 514:102–106
Knutson SK, Wigle TJ, Warholic NM, Sneeringer CJ, Allain CJ et al (2012) A selective inhibitor of EZH2 blocks H3K27 methylation and kills mutant lymphoma cells. Nat Chem Biol 8:890–896
McCabe MT, Ott HM, Ganji G, Korenchuk S, Thompson C et al (2012) EZH2 inhibition as a therapeutic strategy for lymphoma with EZH2-activating mutations. Nature 492:108–112
Copeland RA (2013) Molecular pathways: protein methyltransferases in cancer. Clin Cancer Res 19:6344–6350
Wee S, Dhanak D, Li H, Armstrong SA, Copeland RA et al (2014) Targeting epigenetic regulators for cancer therapy. Ann N Y Acad Sci 1309:30–36
Bush EW, McKinsey TA (2010) Protein acetylation in the cardiorenal axis: the promise of histone deacetylase inhibitors. Circ Res 106:272–284
West AC, Johnstone RW (2014) New and emerging HDAC inhibitors for cancer treatment. J Clin Invest 124:30–39
Xie M, Kong Y, Tan W, May H, Battiprolu PK et al (2014) Histone deacetylase inhibition blunts ischemia/reperfusion injury by inducing cardiomyocyte autophagy. Circulation 129:1139–1151
van Rooij E, Purcell AL, Levin AA (2012) Developing microRNA therapeutics. Circ Res 110:496–507
Thum T (2012) MicroRNA therapeutics in cardiovascular medicine. EMBO Mol Med 4:3–14
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Marín-García, J., Akhmedov, A.T. Epigenetics of the failing heart. Heart Fail Rev 20, 435–459 (2015). https://doi.org/10.1007/s10741-015-9483-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10741-015-9483-x