Abstract
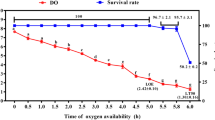
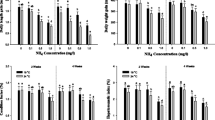
The effects of chronic and periodic peaks of un-ionised ammonia (UIA-N) exposure on eye health and cataract formation in juvenile Atlantic halibut, Hippoglossus hippoglossus, were examined. Fish with mean initial weight 51.7 g (SD 13.2) were exposed to five treatments consisting of control group and three groups (ChronicLow, ChronicMedium and ChronicHigh,) chronically exposed with UIA-N of 0.06, 0.12 to 0.17 mg/l, respectively, for 62 days at 11.9°C, pH 8.0 and salinity 34‰. Furthermore, a fifth group (HighPulse) was exposed to the same high levels as ChronicHigh for a short daily period (peak of 15 mg/l 30 min after exposure, 10 mg/l 1 h after exposure and 1.2 mg/l 3 h after exposure). In the subsequent period of the experimental study (from day 63 until day 100), no ammonia was added to the water. Mean weights were significantly lower in groups exposed to chronically high ambient ammonia concentrations compared to corresponding control group throughout the experimental period. The sampled fish exhibited signs of mild cataract formation, although the results showed no clear evidence that the ammonia treatments contributed to differences. Minor differences were found in measured muscle free amino acids, which could be used to explain potential changes in buffering capacity. The eye histidine status differed significantly at day 62, and osmotic differences in the eye lenses (measured as differences in N-acetyl histidine) were found in all group exposed to chronic levels of ammonia.



Similar content being viewed by others
References
Baslow MH (1998) Function of the N-acetyl-l-histidine system in the vertebrate eye. Evidence in support of a role as a molecular water pump. J Mol Neurosci 10:193–208
Bjerkås E, Sveier H (2006) The influence of nutritional and environmental factors on osmoregulation and cataracts in Atlantic salmon (Salmo salar L.). Aquaculture 235:101–122
Bjerkås E, Bjerkås I, Moksness E (1998) An outbreak of cataract with lens rupture and nuclear extrusion in wolffish (Anarhicas spp.). Vet Opthalmol 1:9–15
Bjerkås E, Bjørnestad E, Breck O, Waagbø R (2001) Water temperature regimes affect cataract development in smolting salmon, Salmo salar L. J Fish Dis 24:281–291
Bjerkås E, Breck O, Waagbø R (2006) The role of nutrition in cataract formation in farmed fish. CAB Reviews: Perspectives in Agriculture, Veterinary Science, Nutrition and Natural Resources 1:1–16
Bjerkås E, Waagbø R, Sveier H, Breck O, Bjerkås I, Bjørnestad E, Maage A (1996) Cataract development in Atlantic salmon (Salmo salar L) in fresh water. Acta Vet Scand 37:351–360
Breck O (2004) Histidine nutrition and cataract development in Atlantic salmon Salmo salar L. PhD thesis, University of Bergen
Breck O, Bjerkås E, Campbell P, Arnesen P, Haldorsen P, Waagbø R (2003) Cataract preventative role of mammalian blood meal, histidine, iron and zinc in diets for Atlantic salmon (Salmo salar L.) of different strains. Aquac Nutr 9:341–350
Breck O, Bjerkås E, Campbell P, Rhodes JD, Sanderson J, Waagbø R (2005a) Histidine nutrition and genotype affect cataract development in Atlantic salmon, Salmo salar L. J Fish Dis 28:357–371
Breck O, Bjerkås E, Sanderson J, Waagbø R, Campbell P (2005b) Dietary histidine affects lens protein turnover and synthesis of N-acetylhistidine in Atlantic salmon (Salmo salar L.) undergoing parr–smolt transformation. Aquac Nutr 11:321–332
Buentello JA, Gatlin DM (2002) Preliminary observations of the effects of water hardness on free taurine and other amino acids in plasma and muscle of channel catfish. North Am J Aquac 64:95–102
Cullen AP, Monteith-McMaster CA, Sivak JG (1994) Lenticular changes in rainbow trout following chronic exposure to UV radiation. Curr Eye Res 13:731–737
Foss A, Evensen TH, Vollen T, Oiestad V (2003) Effects of chonic ammonia exposure on growth and food conversion efficiency in juvenile spotted wolffish. Aquaculture 228:215–224
Foss A, Siikavuopio SI, Sæther BS, Evensen TH (2004) Effect of chronic ammonia exposure on growth in juvenile Atlantic cod. Aquaculture 237:179–189
Handy RD, Poxton MG (1993) Nitrogen pollution in mariculture: toxicity and excretion of nitrogenous compounds by marine fish. Rev Fish Biol Fish 3:205–241
Hiroshi H, Murai T (1994) White muscle of masu salmon, Onchorhynchus masou, smolts possesses a strong buffering capacity due to high level of anserine. Fish Physiol Biochem 13:285–293
Johansson O, Wedborg M (1980) Ammonia-ammonium equilibrium in seawater at temperatures between 5 and 25 degrees C. J Sol Chem 9:37–44
Kim JD, Lall SP (2000) Amino acid composition of whole body tissue of Atlantic halibut (Hippoglossus hippoglossus), yellowtail flounder_(Pleuronectes ferruginea) and Japanese flounder (Paralichthys oliÍaceus). Aquaculture 187:367–373
Leung KMY, Chu JCW, Wu RSS (1999) Effects of body weight, water temperature and ration size on ammonia excretion by the areolated grouper (Epinephelus areolatus) and mangrove snapper (Lutjanus argentimaculatus). Aquaculture 170:215–227
Munakata A, Aida K, Amando M, Ikuta K, Kitamura S, Ogata H (2000) Changes in histidine and anserine levels in hatchery-reared masu salmon parr after release in a river. J World Aquac Soc 31:274–278
NRC (1993) Nutrient requirements of fish. National Academy Press, Washington
O’Dowd JJ, Cairns MT, Trainor M, Robins DJ, Miller DJ (1990) Analysis of carnosine, homocarnosine and other histidyl derivatives in rat brain. J Neurochem 55:446–452
Ogata HY, Konno S, Silverstein JT (1998) Muscular buffering capacity of the parr and smolts in Oncorhynchus masou. Aquaculture 168:303–310
Pankhurst NW, Montgomery JC (1994) Uncoupling visual and the somatic growth in the rainbow trout Oncorhyncus mykiss. Brain Behav Evol 34:861–863
Person-Le Ruyet J, Lamers A, Le Roux A, Sévère A, Boeuf G, Mayer-Gostan N (2003) Long-term ammonia exposure of turbot: effects on plasma parameters. J Fish Biol 62:879–894
Rasmussen RS, Korsgaard B (1998) Ammonia and urea in plasma of juvenile turbot (Scophthalmus maximus) in response to external ammonia. Comp Biochem Physiol 120A:163–168
StatSoft, Inc. (2007) STATISTICA (data analysis software system), version 8. http://www.statsoft.com
Takeuchi T (2001) A review of feed development for early life stages of marine finfish in Japan. Aquaculture 200:203–222
Treasurer JW, Cox D, Wall T (2007) Epidemiology of blindness and cataracts in cage reared ongrown Atlantic halibut. Aquaculture 271:77–84
Valtonen ET, Koskivaara M (1994) Relationships between the parasites of some wild and cultured fishes in two lakes and a fish farm in Central Finland. Int J Parasitol 24:109–118
Waagbø R, Sveier H, Breck O, Bjornestad E, Maage A, Bjerkås E (1998) Cataract formation in smolting Atlantic salmon, Salmo salar, fed low and high energy diets. Bull Europ Ass Fish Pathl 18:201–205
Waagbø R, Hamre K, Bjerkås E, Berge R, Wathne E, Lie O, Tortensen B (2003) Cataract formation in Atlantic salmon, Salmo salar L., smolt relative to dietary pro- and antioxidants and lipid level. J Fish Dis 26:213–229
Waagbø R, Hosfeld CD, Fivelstad S, Olsvik PA, Breck O (2008) The impact of variable water gases on cataract formation, muscle and lens free amino acids, and lens antioxidant enzyme and heat shock protein transcription in smolting Atlantic salmon, Salmo salar L. Comp Biochem Physiol A149:396–404
Waagbø R, Tröße C, Koppe W, Fontanillas R, Breck O (2010) Dietary histidine supplementation prevents cataract development in adult Atlantic salmon, Salmo salar L in sea. Brit J Nutr 104:1460–1470
Wade MA, Tucker HN (1998) Antioxidant characteristics of l-histidine. J Nutr Biochem 9:308–315
Wagner E, Miller S, Bosakowski T (1995) Ammonia excretion by rainbow trout over a 24 hour period at two densities during oxygen injection. Prog Fish Cult 57:199–205
Wall T, Bjerkås E (1999) A simplified method of scoring cataracts in fish. Bull Europ Ass Fish Pathol 19:162–165
Whitfield M (1974) The hydrolysis of ammonia ions in sea water—a theoretical study. J Mar Biol Assoc UK 54:565–580
Williams DL, Wall EA, Brandson E, Hopcroft T, Poole A, Brancker WM (1995) Preliminary findings of ophthalmological abnormalities in farmed halibut. Vet Rec Rec 10:612–660
Yamada S, Furuichi M (1990) Nα-Acetylhistidine metabolism in fish-1. Identification of Nα-acetylhistidine in the heart of rainbow trout Salmo gairdneri. Comp Physiol Biochem 97B:539–541
Yamada S, Kawashima K, Baba K, Oku T, Ando S (2009) Occurrence of novel acylated amino acids Nα-acetylhistidine, in skeletal muscle of freshwater fish and other ectothermic vertebrates. Comp Biochem Phsyiol 152B:282–286
Zar JH (1996) Biostatistical analysis, 3rd edn. Prentice-Hall, Englewood Cliffs
Acknowledgments
The authors wish to thank engineers Mrs Anita Birkenes and Torill Berg for their technical assistance during the experimental period. This study was financed by the Norwegian Research Council (No. 180088/120).
Author information
Authors and Affiliations
Corresponding author
Additional information
An erratum to this article can be found at http://dx.doi.org/10.1007/s10695-011-9545-5.
Rights and permissions
About this article
Cite this article
Liakonis, K.M., Waagbø, R., Foss, A. et al. Effects of chronic and periodic exposures to ammonia on the eye health in juvenile Atlantic halibut (Hippoglossus hippoglossus). Fish Physiol Biochem 38, 421–430 (2012). https://doi.org/10.1007/s10695-011-9521-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10695-011-9521-0