Abstract
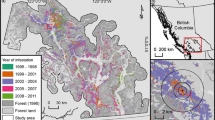
The challenge of observing interactions between plant pathogens, their hosts, and environmental heterogeneity across multiple spatial scales commonly limits our ability to understand and manage wildland forest epidemics. Using the forest pathogen Phytopthora ramorum as a case study, we established 20 multiscale field sites to analyze how host-pathogen-environment relationships vary across spatial scales of observation in a wildland pathosystem. We developed statistical models of disease intensity across five nested levels of spatial aggregation, from an individual host through four broader spatial extents of observation. Analyses were conducted from two spatial perspectives: a focal view, where disease intensity at one scale was examined as a function of broader-scale landscape conditions, and an aggregate view, where disease intensity and landscape conditions was observed at the same scale of spatial aggregation. For each perspective, separate models were developed to compare direct field measurements of host density versus less expensive remotely sensed estimates of host habitat as predictors of disease in landscape-scale studies. From both perspectives, models using direct measurements of host density performed better than models using remotely sensed estimates of host habitat across all four spatial extents. We found no significant difference in model performance at the individual level. From the focal view, the performance of host density models declined with increasing spatial extent, whereas the performance of host habitat models improved with spatial extent. These results illustrate how the scale of observation – both spatial extent and measurement detail – can influence conclusions drawn from epidemiological models of wildland pathosystems.






Similar content being viewed by others
References
Akaike, H. (1974). A new look at the statistical model identification. IEEE Transactions on Automatic Control, 19(6), 716–723.
Anacker, B. L., Rank, N. E., Hüberli, D., Garbelotto, M., Gordon, S., Harnik, T., & Meentemeyer, R. K. (2008). Susceptibility to Phytophthora ramorum in a key infectious host: landscape variation in host genotype, host phenotype, and environmental factors. New Phytologist, 177(3), 756–766.
APHIS. (2013). Plant health: Phytophthora ramorum/sudden oak death. USDA Animal and Plant Health Inspection Service, Washington D.C. Retrieved August 29, 2010, from http://www.aphis.usda.gov/plant_health/plant_pest_info/pram/index.shtml.
Barton, K. (2013). MuMIn: Multi-model inference. Retrieved from http://cran.r-project.org/package=MuMIn.
Bates, D., Maechler, M., Bolker, B., & Walker, S. (2013). lme4: Linear mixed-effects models using Eigen and S4. Retrieved from http://cran.r-project.org/package=lme4.
Bolker, B. M., Brooks, M. E., Clark, C. J., Geange, S. W., Poulsen, J. R., Stevens, M. H. H., & White, J.-S. S. (2009). Generalized linear mixed models: a practical guide for ecology and evolution. Trends in Ecology & Evolution, 24(3), 127–135.
Burdon, J. J., & Chilvers, G. A. (1982). Host Density as a Factor in Plant Disease Ecology. Annual Review of Phytopathology, 20(1), 143–166.
Burdon, J. J., Thrall, P. H., & Ericson, A. L. (2006). The current and future dynamics of disease in plant communities. Annual Review of Phytopathology, 44, 19–39.
Burdon, J. J., Thrall, P., & Ericson, L. (2013). Genes, communities & invasive species: understanding the ecological and evolutionary dynamics of host-pathogen interactions. Current Opinion in Plant Biology, 16(4), 400–405.
Burnham, K., & Anderson, D. (2002). Model selection and multi-model inference: a practical information-theoretic approach (2nd ed.). New York: Springer-Verlag.
Burnham, K. P., Anderson, D. R., & Huyvaert, K. P. (2010). AIC model selection and multimodel inference in behavioral ecology: some background, observations, and comparisons. Behavioral Ecology and Sociobiology, 65(1), 23–35.
Chave, J. (2013). The problem of pattern and scale in ecology: what have we learned in 20 years? Ecology Letters. doi:10.1111/ele.12048.
Cobb, R. C., Meentemeyer, R. K., & Rizzo, D. M. (2010). Apparent competition in canopy trees determined by pathogen transmission rather than susceptibility. Ecology, 91(2), 327–333.
Cobb, R. C., Filipe, J. A. N., Meentemeyer, R. K., Gilligan, C. A., & Rizzo, D. M. (2012). Ecosystem transformation by emerging infectious disease: loss of large tanoak from California forests. Journal of Ecology. doi:10.1111/j.1365-2745.2012.01960.x.
Condeso, T. E., & Meentemeyer, R. K. (2007). Effects of landscape heterogeneity on the emerging forest disease sudden oak death. Journal of Ecology, 95(2), 364–375.
Cottam, G., & Curtis, J. (1956). The use of distance measures in phytosociological sampling. Ecology, 37(3), 451–460.
Cushman, J. H., & Meentemeyer, R. K. (2008). Multi-scale patterns of human activity and the incidence of an exotic forest pathogen. Journal of Ecology, 96(4), 766–776.
Davidson, J. M., Wickland, A. C., Patterson, H. A., Falk, K. R., & Rizzo, D. M. (2005). Transmission of Phytophthora ramorum in mixed-evergreen forest in California. Phytopathology, 95(5), 587–596.
Davidson, J. M., Patterson, H. A., & Rizzo, D. M. (2008). Sources of inoculum for Phytophthora ramorum in a redwood forest. Phytopathology, 98(8), 860–866.
Dillon, W. W., Meentemeyer, R. K., Vogler, J. B., Cobb, R. C., Metz, M. R., & Rizzo, D. M. (2013). Range-wide threats to a foundation tree species from disturbance interactions. Madrono, 60(2), 139–150.
Dinoor, A., & Eshed, N. (1984). The Role and Importance of Pathogens in Natural Plant Communities. Annual Review of Phytopathology, 22(1), 446–466.
Ellis, A. M., VaclavÌk, T., Meentemeyer, R. K., & Václavík, T. (2010). When is connectivity important? A case study of the spatial pattern of sudden oak death. Oikos, 119(3), 485–493.
Englander, L., Browning, M., & Tooley, P. W. (2006). Growth and sporulation of Phytophthora ramorum in vitro in response to temperature and light. Mycologia, 98(3), 365–373.
Gelman, A., & Hill, J. (2007). Data analysis using regression and multilevel/hierarchical models. New York, NY: Cambridge University Press.
Gilbert, G. S. (2002). Evolutionary ecology of plant diseases in natural ecosystems. Annual Review of Phytopathology, 40(120), 13–43.
Haas, S. E., Hooten, M. B., Rizzo, D. M., & Meentemeyer, R. K. (2011). Forest species diversity reduces disease risk in a generalist plant pathogen invasion. Ecology Letters, 14(11), 1108–1116.
Hansen, E. M., Kanaskie, A., Prospero, S., McWilliams, M., Goheen, E. M., Osterbauer, N., & Sutton, W. (2008). Epidemiology of Phytophthora ramorum in Oregon tanoak forests. Canadian Journal of Forest Research, 38(5), 1133–1143.
Holdenrieder, O., Pautasso, M., Weisberg, P. J., & Lonsdale, D. (2004). Tree diseases and landscape processes: the challenge of landscape pathology. Trends in Ecology & Evolution, 19(8), 446–452.
Hüberli, D., Hayden, K. J., Calver, M., & Garbelotto, M. (2011). Intraspecific variation in host susceptibility and climatic factors mediate epidemics of sudden oak death in western US forests. Plant Pathology, 61(3), 579–592.
Jeger, M. J., Pautasso, M., Holdenrieder, O., & Shaw, M. W. (2007). Modelling disease spread and control in networks: implications for plant sciences. The New Phytologist, 174(2), 279–297.
Kauffman, M. J., & Jules, E. S. (2006). Heterogeneity Shapes Invasion: Host Size And Environment Influence Susceptibility To A Nonnative Pathogen. Ecological Applications, 16(1), 166–175.
Mascheretti, S., Croucher, P. J. P., Vettraino, A., Prospero, S., & Garbelotto, M. (2008). Reconstruction of the Sudden Oak Death epidemic in California through microsatellite analysis of the pathogen Phytophthora ramorum. Molecular Ecology, 17(11), 2755–2768.
Meentemeyer, R., Rizzo, D., Mark, W., & Lotz, E. (2004). Mapping the risk of establishment and spread of sudden oak death in California. Forest Ecology and Management, 200(1–3), 195–214.
Meentemeyer, R. K., Rank, N. E., Shoemaker, D. A., Oneal, C. B., Wickland, A. C., Frangioso, K. M., & Rizzo, D. M. (2008a). Impact of sudden oak death on tree mortality in the Big Sur ecoregion of California. Biological Invasions, 10(8), 1243–1255.
Meentemeyer, R. K., Rank, N. E., Anacker, B. L., Rizzo, D. M., & Cushman, J. H. (2008b). Influence of land-cover change on the spread of an invasive forest pathogen. Ecological Applications, 18(1), 159–171.
Meentemeyer, R. K., Haas, S. E., & Václavík, T. (2012). Landscape Epidemiology of Emerging Infectious Diseases in Natural and Human-Altered Ecosystems. Annual Review of Phytopathology, 50, 379–402.
Metz, M. R., Frangioso, K. M., Meentemeyer, R. K., & Rizzo, D. M. (2011). Interacting disturbances: wildfire severity affected by stage of forest disease invasion. Ecological Applications, 21(2), 313–320.
Metz, M. R., Frangioso, K. M., Wickland, A. C., Meentemeyer, R. K., & Rizzo, D. M. (2012). An emergent disease causes directional changes in forest species composition in coastal California. Ecosphere, 3(10), art86.
Morrison, J. (1996). Infection of Juncus dichotomus by the smut fungus Cintractia junci: an experimental field test of the effects of neighbouring plants, environment, and host plant genotype. Journal of Ecology, 84(5), 691–702.
Mundt, C. C., & Sackett, K. E. (2012). Spatial scaling relationships for spread of disease caused by a wind-dispersed plant pathogen. Ecosphere, 3(3), 1–10.
O’Hara, R. B., & Kotze, D. J. (2010). Do not log-transform count data. Methods in Ecology and Evolution, 1(2), 118–122.
Ostfeld, R. S., Glass, G. E., & Keesing, F. (2005). Spatial epidemiology: an emerging (or re-emerging) discipline. Trends in Ecology & Evolution, 20(6), 328–336.
Plantegenest, M., Le May, C., & Fabre, F. (2007). Landscape epidemiology of plant diseases. Journal of the Royal Society Interface/the Royal Society, 4(16), 963–972.
R Core Team. (2013). R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. http://www.r-project.org/.
Real, L., & McElhany, P. (1996). Spatial Pattern and Process in Plant-Pathogen Interactions. Ecology, 77(4), 1011–1025.
Rizzo, D. M., & Garbelotto, M. (2003). Sudden oak death: endangering California and Oregon forest ecosystems. Frontiers in Ecology and the Environment, 1(4), 197–204.
Rizzo, D. M., Garbelotto, M., & Hansen, E. M. (2005). Phytophthora ramorum: integrative research and management of an emerging pathogen in California and Oregon forests. Annual Review of Phytopathology, 43(1), 309–335.
Swiecki, T., & Bernhardt, E. (2007). Increasing Distance from California Bay Laurel Reduces the Risk and Severity of Phytophthora ramorum Canker in Coast Live Oak. … Science Symposium, Santa Rosa, California (pp. 181–194). Albany, California, USA. http://www.fs.fed.us/psw/publications/documents/psw_gtr214/psw_gtr214_181-194_swiecki.pdf.
Tooley, P., Browning, M., & Berner, D. (2008). Recovery of Phytophthora ramorum following exposure to temperature extremes. Plant Disease, 92, 431–437. doi:10.1094/PDIS-92-3-0431.
Václavík, T., Kupfer, J. A., & Meentemeyer, R. K. (2012). Accounting for multi-scale spatial autocorrelation improves performance of invasive species distribution modelling (iSDM). Journal of Biogeography, 39(1), 42–55.
Wiens, J. A. (1989). Spatial scaling in ecology. Functional Ecology, 3(4), 385–397.
Acknowledgments
We extend our gratitude to Monica Dorning, Richard Cobb, and anonymous reviewers for providing constructive critiques on previous versions of this manuscript; Amelia Johnson and Steve Johnston for their assistance with field data collection; the Sonoma State University Preserve System Fairfield-Osborne Preserve, the Sonoma Mountain Ranch Preservation Foundation, the Sonoma Agriculture and Open Space District, as well as multiple private land owners for granting us access to their lands to collect the data used here. This research was supported in part by a grant from the National Science Foundation (DEB-1115720) as part of the joint NSF-NIH Ecology of Infectious Disease program and National Science Foundation Research Experience for Undergraduates Program (09-598) Award Supplement (0622677). We also gratefully acknowledge financial support from the United States Department of Agriculture Forest Service-Pacific Southwest Research Station.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
Correlation plots and coefficients between bay laurel density (c.ustem.dens.X) and proportion of host habitat (pct.hh.X) across the four spatial extents of the multiscale study sites. The number at the end of each variable indicates the extent at which it was measured/calculated, e.g. ‘pct.hh.60’ is the proportion of host habitat calculated for the 60-m extent
Rights and permissions
About this article
Cite this article
Dillon, W.W., Haas, S.E., Rizzo, D.M. et al. Perspectives of spatial scale in a wildland forest epidemic. Eur J Plant Pathol 138, 449–465 (2014). https://doi.org/10.1007/s10658-013-0376-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10658-013-0376-3