Abstract
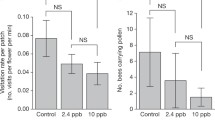
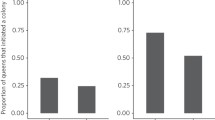
Bumblebees and other pollinators provide a vital ecosystem service for the agricultural sector. Recent studies however have suggested that exposure to systemic neonicotinoid insecticides in flowering crops has sub-lethal effects on the bumblebee workforce, and hence in reducing queen production. The mechanism behind reduced nest performance, however, remains unclear. Here we use Radio Frequency Identification (RFID) technology to test whether exposure to a low, field realistic dose (0.7 ppb in sugar water and 6 ppb in pollen) of the neonicotinoid imidacloprid, reduces worker foraging efficiency. Whilst the nectar foraging efficiency of bees treated with imidacloprid was not significantly different than that of control bees, treated bees brought back pollen less often than control bees (40 % of trips vs 63 % trips, respectively) and, where pollen was collected, treated bees brought back 31 % less pollen per hour than controls. This study demonstrates that field-realistic doses of these pesticides substantially impacts on foraging ability of bumblebee workers when collecting pollen, and we suggest that this provides a causal mechanism behind reduced queen production in imidacloprid exposed colonies.

Similar content being viewed by others
References
Akaike H (1974) A new look at the statistical model identification. IEEE Trans Autom Control 19:716–723
Aliouane Y, El Hassani AK, Gary V, Armengaud C, Lambin M, Gauthier M (2009) Subchronic exposure of honeybees to sublethal doses of pesticides: effects on behaviour. Environ Toxicol Chem 28:113–122
Bonmatin JM, Moineau I, Charvet R, Fléché C, Colin ME, Bengsch ER (2003) A LC/APCI-MS/MS method for analysis of imidacloprid in soils, in plants and in pollens. Anal Chem 75:2027–2033
Bortolotti L, Montanari R (2003) Effects of sub-lethal imidacloprid doses on the homing rate and foraging activity of honey bees. Bull Insectol 56:63–67
Carvell C, Roy DB, Smart SM, Pywell RF, Preston CD, Goulson D (2006) Declines in forage availability for bumblebees at a national scale. Biol Conserv 132:481–489
Cresswell JE (2011) A meta-analysis of experiments testing the effects of a neonicotinoid insecticide (imidacloprid) on honey bees. Ecotoxicology 20:149–157
Decourtye A, Devillers J, Cluzeau S, Charreton M, Pham-Delègue MH (2004) Effects of imidacloprid and deltamethrin on associative learning in honeybees under semi-field and laboratory conditions. Ecotoxicol Environ Saf 57:410–419
DEFRA (2012) Farming statistics: final crop areas, yields, livestock populations and agricultural workforce. Farming Statistics, Department for Environment, Food and Rural Affairs, London
Desneux N, Decourtye A, Delpuech JM (2007) The Sublethal Effects of Pesticides on Beneficial Arthropods. Annu Rev Entomol 52:81–106
Di Prisco G, Cavaliere V, Annoscia D, Varricchio P, Caprio E, Nazzi F, Gargiulo G, Pennacchio F (2013) Neonicotinoid clothianidin affects insect immunity and promotes replication of a viral pathogen in honey bees. PNAS 201314923 110(46):18466–18471. doi:10.1073/pnas.1314923110
EFSA (2012) Statement on the findings in recent studies investigating sub-lethal effects in bees of some neonicotinoids in consideration of the uses currently authorised in Europe. EFSA J 10:2752
Elston C, Thompson HM, Walters KFA (2013) Sub-lethal effects of thiamethoxam, a neonicotinoid pesticide, and propiconazole, a DMI fungicide, on colony initiation in bumblebee (Bombus terrestris) micro-colonies. Apidologie 44:563–574
European Commission (2013) Bee health: EU-wide restrictions on pesticide use to enter into force. European Commission, Brussels
Garthwaite DG, Barker I, Parrish G, Smith L, Chippindale C, Pietravalle S (2010) Pesticide usage survey report 235-Arable crops in the United Kingdom. DEFRA, London
Gill RJ, Ramos-Rodriguez O, Raine NE (2012) Combined pesticide exposure severely affects individual and colony-level traits in bees. Nature 491:105–108. doi:10.1038/nature11585
Goulson D (2010) Bumblebees: behaviour, ecology and conservation. Oxford University Press, Oxford
Goulson D, Peat J, Stout JC, Tucker J, Darvill B, Derwent LC, Hughes WOH (2002) Can alloethism in workers of the bumblebee, Bombus terrestris, be explained in terms of foraging efficiency? Anim Behav 64:123–130
Goulson D, Lye GC, Darvill B (2008) Decline and conservation of bumblebees. Annu Rev Entomol 53:191–208
Goulson D, Lepais O, O’Connor S, Osborne JL, Sanderson R, Cussans J, Goffe L, Darvill B (2010) Effects of land use at a landscape scale on bumblebee nest density and survival. J Appl Ecol 47:1207–1215
Harder LD (1990) Behavioural responses by bumble bees to variation in pollen availability. Oecologia 85:41–47
Hayter KE, Cresswell JE (2006) The influence of pollinator abundance on the dynamics and efficiency of pollination in agricultural Brassica napus: implications for landscape-scale gene dispersal. J Appl Ecol 43:1196–1202
Henry M, Béguin M, Requier F, Rollin O, Odoux JF, Aupinel P, Aptel J, Tchamitchian S, Decourtye A (2012) A common pesticide decreases foraging success and survival in honey bees. Science 336:348–350
Klein AM, Vaissière BE, Cane JH, Steffan-Dewenter I, Cunningham SA, Kremen C, Tscharntke T (2007) Importance of pollinators in changing landscapes for world crops. Proc R Soc B 274:303–313
Krupke CH, Hunt GJ, Eitzer BD, Andino G, Given K (2012) Multiple Routes of Pesticide Exposure for Honey Bees Living Near Agricultural Fields. PLoS ONE 7. doi:10.1371/journal.pone.0029268
Laycock I, Lenthall KM, Barratt AT, Cresswell JE (2012) Effects of imidacloprid, a neonicotinoid pesticide, on reproduction in worker bumble bees (Bombus terrestris). Ecotoxicology 21:1937-1945
Matsumoto T (2013) Reduction on homing flights in the honey bee Apis mellifera after a sublethal does of neonicotinoid insecticides. Bull Insectol 66:1–9
McGregor SE (1976) USDA Agriculture Handbook No. 496. Insect pollination of cultivated crops. USDA, Washington DC
Molet M, Chittka L, Stelzer RJ, Streit S, Raine NE (2008) Colony nutritional status modulates worker responses to foraging recruitment pheromone in the bumblebee Bombus terrestris. Behav Ecol Sociobiol 62:1919–1926
Müller C, Schmid Hempel P (1992) Correlates of reproductive success among field colonies of Bombus lucorum: the importance of growth and parasites. Ecolo Entomol 17:343–353
Nauen R, Ebbinghaus-Kintscher U, Salgado VL, Kaussmann M (2003) Thiamethoxam is a neonicotinoid precursor converted to clothianidin in insects and plants. Pestic Biochem Physiol 76:55–69
Ohashi K, D’Souza D, Thomson JD (2010) An automated system for tracking and identifying individual nectar foragers at multiple feeders. Behav Ecol Sociobiol 64:891–897
Peat J, Goulson D (2005) Effects of experience and weather on foraging rate and pollen versus nectar collection in the bumblebee, Bombus terrestris. Behav Ecol Sociobiol 58:152–156
Pollak P (2011) Fine chemicals: the industry and the business. Wiley, Hoboken
Raine NE, Chittka L (2007) Pollen foraging: learning a complex motor skill by bumblebees (Bombus terrestris). Die Naturwissenschaften 94:459–464
Robinson EJH, Richardson TO, Sendova-Franks AB, Feinerman O, Franks NR (2009) Radio tagging reveals the roles of corpulence, experience and social information in ant decision making. Behav Ecol Sociobiol 63:627–636
Stelzer RJ, Chittka L, Carlton M, Ings TC (2010) Winter active bumblebees (Bombus terrestris) achieve high foraging rates in Urban Britain. PLoS ONE 5. doi:10.1371/journal.pone.0009559
Streit S, Bock F, Pirk CWW, Tautz J (2003) Automatic life-long monitoring of individual insect behaviour now possible. Zoology 106:169–171
Sumner S, Lucas E, Barker J, Isaac N (2007) Radio-tagging technology reveals extreme nest-drifting behaviour in a eusocial insect. Curr Biol 17:140–145
Thompson H, Harrington P, Wilkins W, Pietravalle S, Sweet D, Jones A (2013) Effects of neonicotinoid seed treatments on bumble bee colonies. Food Environ Res Agency Rep. http://fera.co.uk/ccss/documents/defraBumbleBeeReportPS2371V4a.pdf. Accessed 29 Oct 2013
Van Der Steen JJM (2008) Intection and transmission of Nosema bombie in Bombus terrestris colonies and its effect on hibernation, mating and colony founding. Apidologie 39:273–282
Whitehorn PR, O’Connor S, Wackers FL, Goulson D (2012) Neonicotinoid pesticide reduces bumble bee colony growth and queen production. Science 336:351–352
Williamson SM, Wright GA (2013) Exposure to multiple cholinergic pesticides impairs olfactory learning and memory in honeybees. J Exp Biol 216:1799–1807
Yang EC, Chuang YC, Chen YL, Chang LH (2008) Abnormal foraging behaviour induced by sublethal dosage of imidacloprid in the honey bee (Hymenoptera: Apidae). J Econ Entomol 101:1743–1748
Yang EC, Chang HC, Wu WY, Chen YW (2012) Impaired olfactory associative behaviour of honeybee workers due to contamination of imidacloprid in the larval stage. PLoS ONE 7. doi:10.1371/journal.pone.0049472
Acknowledgments
We would like to thank Aaron Hamilton and Stephan Hamilton for their assistance in data collection. We would also like to thank Jeroen Minderman and Timothy Paine for their advice on analysis and James Weir for his technical support. Finally we would like to thank the Natural Environment Research Council and the Economic and Social Research Council for their financial support.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Feltham, H., Park, K. & Goulson, D. Field realistic doses of pesticide imidacloprid reduce bumblebee pollen foraging efficiency. Ecotoxicology 23, 317–323 (2014). https://doi.org/10.1007/s10646-014-1189-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-014-1189-7