Abstract
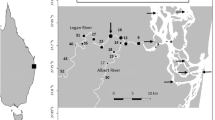
Acoustic telemetry was used to examine the size of daily activity space, small-scale movement patterns, and water quality preferences of juvenile bull sharks in the Caloosahatchee River, Florida. Movement pattern analysis included rate of movement, swimming depth, linearity, direction, tidal influence, diel pattern, and correlation with environmental variables. Manual tacking occurred before and after a large freshwater influx which divided the sharks into two groups based on movement patterns. The first group displayed increased rate of movement, distance traveled, and space utilization at night, and movements correlated with salinity, temperature, and dissolved oxygen. The second group had an increased rate of movement, distance traveled, and space utilization during the day, and movements correlated with temperature, dissolved oxygen, turbidity and pH. These juvenile bull sharks displayed distinct diel movement patterns that were influenced by physical factors, which may account for the distribution of this top-level predator in the Caloosahatchee River.




Similar content being viewed by others
References
Ackerman JT, Kondratieff MC, Matern SA, Cech JJ Jr (2000) Tidal influence on spatial dynamics of leopard sharks, Triakis semifasciata, in Tomales Bay, California. Environ Biol Fishes 58:33–43. doi:10.1023/A:1007657019696
Albanese B, Angermeier PL, Dorai-Raj S (2004) Ecological correlates of fish movement in a network of Virginia streams. Can J Fish Aquat Sci 61:857–869. doi:10.1139/f04-096
Barnes T (2005) Caloosahatchee Estuary conceptual ecological model. Wetlands 25:884–897
Bass AJ, D’Aubrey JD, Kistnasamy N (1973) Sharks of the east coast of southern Africa. 1. The genus Carcharhinus (Carcharhinidae). Oceanographic Research Institute (Durban). Investig Rep 33:1–168
Bell WJ, Kramer E (1979) Search for anemotactic orientation of cockroaches. J Insect Physiol 25:631–640. doi:10.1016/0022-1910(79)90112-4
Branstetter S (1981) Biological notes on the sharks of the north central Gulf of Mexico. Contrib Mar Sci 24:13–34
Brenden TO, Murphy BR, Hallerman EM (2006) Effect of discharge on daytime habitat use and selection by muskellunge in the New River, Virginia. Trans Am Fish Soc 135:1546–1558. doi:10.1577/T05-256.1
Compagno LJV (1984) FAO Species catalogue. Vol 4. Sharks of the world: an annotated and illustrated catalogue of shark species known to date. Part 2. Carcharhiniformes. FAO Fish Synop 125:251–655
Curtis TH (2008) Distribution, movements, and habitat use of bull sharks (Carcharhinus leucas, Müller and Henle 1839) in the Indian River Lagoon system, Florida. M.S. thesis, University of Florida, Gainesville, pp. 130
Dresser BK, Kneib RT (2007) Site fidelity and movement patterns of wild subadult red drum, Sciaenops ocellatus (Linnaeus), within a salt marsh-dominated estuarine landscape. Fish Manag Ecol 14:183–190. doi:10.1111/j.1365-2400.2007.00526.x
Flannery MS, Peebles EB, Montgomery RT (2002) A percent-of-flow approach for managing reductions of freshwater inflows from unimpounded rivers to southwest Florida estuaries. Estuaries 25:1318–1332. doi:10.1007/BF02692227
Gowan C, Fausch KD (2002) Why do foraging stream salmonids move during summer? Environ Biol Fishes 64:139–153. doi:10.1023/A:1016010723609
Grubbs RD, Musick JA, Conrath CL, Romine JG (2007) Long-term movements, migrations, and temporal delineation of a summer nursery for juvenile sandbar sharks in the Chesapeake Bay region. In: McCandless CT, Pratt HL Jr, Kohler NE (eds) Shark nursery grounds of the Gulf of Mexico and East Coast waters of the United States. Am Fish Soc Symp 50:63–86
Gruber SH, Nelson DR, Morrissey JF (1988) Patterns of activity and space utilization of lemon sharks, Negaprion brevirostris, in a shallow Bahamian lagoon. Bull Mar Sci 43:61–76
Harrison TD, Whitfield AK (2006) Temperature and salinity as primary determinants influencing the biogeography of fishes in South African estuaries. Estuar Coast Shelf Sci 66:335–345. doi:10.1016/j.ecss.2005.09.010
Heupel MR (2007) Exiting Terra Ceia Bay: examination of cues stimulating migration from a summer nursery area. In: McCandless CT, Pratt HL Jr, Kohler NE (eds) Shark nursery grounds of the Gulf of Mexico and East Coast waters of the United States. Am Fish Soc Symp 50:265–280
Heupel MR, Simpfendorfer CA (2008) Movements and distribution of young bull sharks (Carcharhinus leucas) in a variable estuarine environment. Aquat Biol 1:277–289. doi:10.3354/ab00030
Holland KN, Lowe CG, Peterson JD, Gill A (1992) Tracking coastal sharks with small boats: Hammerhead shark pups as a case study. Aust J Mar Freshwater Res 43:61–66. doi:10.1071/MF9920061
Hopkins TE, Cech JJ Jr (2003) The influence of environmental variables on the distribution and abundance of three elasmobranchs in Tomales Bay, California. Environ Biol Fishes 66:279–291. doi:10.1023/A:1023907121605
Hooge PN, Eichenlaub WM (2000) Animal movements extension to ArcView. Alaska Biological Center, US Geological Survey, Anchorage
Kanou K, Sano M, Kohno H (2005) Larval and juvenile fishes occurring with flood tides on an intertidal mudflat in the Tama River estuary, central Japan. Ichthyol Res 52:158–164. doi:10.1007/s10228-005-0267-5
Kernohan BJ, Gitzen RA, Millspaugh JJ (2001) Analysis of animal space use and movements. In: Millspaugh JJ, Marzluff JM (eds) Radio Tracking and Animal Populations. Academic, pp. 125–166
Kidder GW, Petersen CW, Preston RL (2006) Energetics of osmoregulation: II. water flux and osmoregulatory work in the euryhaline fish, Fundulus heteroclitus. J Exp Zool Part A Comp Exp Biol 305A:318–327. doi:10.1002/jez.a.252
Klimley AP, Butler SB, Nelson DR, Stull AT (1988) Diurnal movements of scalloped hammerhead sharks, Sphyrna lewini, to and from a seamount in the Gulf of California. J Fish Biol 33:751–761. doi:10.1111/j.1095-8649.1988.tb05520.x
Krumme U (2004) Patterns in tidal migration of fish in a Brazilian mangrove channel as revealed by a split-beam echosounder. Fish Res 70:1–15. doi:10.1016/j.fishres.2004.07.004
Kupschus S, Tremain D (2001) Association between fish assemblages and environmental factors in nearshore habitats of a subtropical estuary. J Fish Biol 58:1383–1403. doi:10.1111/j.1095-8649.2001.tb02294.x
Martin RA (2005) Conservation of freshwater and euryhaline elasmobranchs: a review. J Mar Biol Assoc U K 85:1049–1073. doi:10.1017/S0025315405012105
Matern SA, Cech JJ Jr, Hopkins TE (2000) Diel movements of bat rays, Myliobatis californica, in Tomales Bay, California: evidence for behavioral thermoregulation? Environ Biol Fishes 58:173–182. doi:10.1023/A:1007625212099
McKibben JN, Nelson DR (1986) Patterns of movement and grouping of gray reef sharks, Carcharhinus amblyrhynchos, at Enewetak, Marshall Islands. Bull Mar Sci 38:89–110
Medved RJ, Marshall JA (1983) Short-term movements of young sandbar sharks, Carcharhinus plumbeus. Bull Mar Sci 33:87–93
Morrissey FJ, Gruber SH (1993a) Habitat selection by juvenile lemon sharks, Negaprion brevirostris. Environ Biol Fishes 38:311–319. doi:10.1007/BF00007524
Morrissey FJ, Gruber SH (1993b) Home range of juvenile lemon sharks, Negaprion brevirostris. Copeia 1993:425–434. doi:10.2307/1447141
Muncy RJ, Wingo WM (1983) Species profiles: life histories and environmental requirements of coastal fishes and invertebrates (Gulf of Mexico) – sea catfish and gafftopsail catfish. U.S. Fish and Wildlife Service, Division of Biological Services, FWS/OBS-82/11.5. U.S. Army Corps of Engineers, TR EL-82-4, pp. 17
Paperno R, Brodie RB (2004) Effects of environmental variables upon the spatial and temporal structure of a fish community in a small, freshwater tributary of the Indian River Lagoon, Florida. Estuar Coast Shelf Sci 61:229–241. doi:10.1016/j.ecss.2004.05.002
Parsons GR, Carlson JK (1998) Physiological and behavioral responses to hypoxia in the bonnethead shark, Sphyrna tiburo: routine swimming and respiratory regulation. Fish Physiol Biochem 19:189–196. doi:10.1023/A:1007730308184
Pillans RD, Franklin CE (2004) Plasma osmolyte concentrations and rectal gland mass of bull sharks Carcharhinus leucas, captured along a salinity gradient. Comp Biochem Physiol A 138:363–371. doi:10.1016/j.cbpb.2004.05.006
Pillans RD, Good JP, Anderson WG, Hazon N, Franklin CE (2005) Freshwater to seawater acclimation of juvenile bull sharks (Carcharhinus leucas): plasma osmolytes and Na+/K+-ATPase activity in gill, rectal gland, kidney and intestine. J Comp Physiol [B] 175:37–44. doi:10.1007/s00360-004-0460-2
Pillans RD, Anderson WG, Good JP, Hyodo S, Takei Y, Hazon N, Franklin CE (2006) Plasma and erythrocyte solute properties of juvenile bull sharks, Carcharhinus leucas, acutely exposed to increasing environmental salinity. J Exp Mar Biol Ecol 331:145–157. doi:10.1016/j.jembe.2005.10.013
Rechisky EL, Wetherbee BM (2003) Short-term movements of juvenile and neonate sandbar sharks, Carcharhinus plumbeus, on their nursery grounds in Delaware Bay. Environ Biol Fishes 68:113–128. doi:10.1023/B:EBFI.0000003820.62411.cb
Sadowsky V (1968) On the measurement of total length of sharks. Zool Anz 181:197–199
Schlosser IJ, Angermeier PL (1995) Spatial variation in demographic processes of lotic fishes: conceptual models, empirical evidence, and implications for conservation. In: Nelson JL (ed) Evolution and the aquatic ecosystem: defining unique units in population conservation. Amer Fish Soc Symp 17:392–401
Schmid T (1988) Age, growth and movement of the Atlantic stingray, Dasyatis sabina, in a Florida coastal lagoon. MS thesis, University of Central Florida, Orlando, FL
Simpfendorfer CA, Greitas GG, Wiley TR, Heupel MR (2005) Distribution and habitat partitioning of immature bull sharks (Carcharhinus leucas) in a Southwest Florida Estuary. Estuaries 28:78–85. doi:10.1007/BF02732755
Snelson FF, Mulligan TJ, Wlliams SE (1984) Food habits, occurrence, and population structure of the bull shark, Carcharhinus leucas, in Florida coastal lagoons. Bull Mar Sci 34:71–80
South Florida Water Management District, Florida Department of Environmental Protection, Florida Department of Agriculture and Consumer Services (2008) Lake Okeechobee Watershed Construction Project, Phase II Technical Plan
Teaf CM (1978) A study of the tidally-oriented movements of the Atlantic stingray, Dasyatis sabina, in Apalachee Bay, Florida. M.S. Thesis, Florida State University, Tallahassee, p 48
Thorson TB, Watson DE, Cowan CM (1966) The status of the freshwater shark of Lake Nicaragua. Copeia 1966:385–402. doi:10.2307/1441058
Thorson TB, Cowan CM, Watson DE (1973) Body fluid solutes of juveniles and adults of the euryhaline bull shark Carcharhinus leucas from freshwater and saline environments. Physiol Zool 46:29–42
Tricas TC, Taylor LR, Naftel G (1981) Diel behavior of the tiger shark, Galeocerdo cuvier, at French Frigate Shoals, Hawaiian Islands. Copeia 1981:904–908. doi:10.2307/1444199
Vaudo JJ, Lowe CG (2006) Movement patterns of the round stingray Urobatis halleri (Cooper) near a thermal outfall. J Fish Biol 68:1756–1766. doi:10.1111/j.0022-1112.2006.01054.x
Vega-Cendejas Ma E, Hernández de Santillana M (2004) Fish community structure and dynamics in a coastal hypersaline lagoon: Rio Lagartos, Yucatan, Mexico. Estuar Coast Shelf Sci 60:285–299. doi:10.1016/j.ecss.2004.01.005
Wiley TR, Simpfendorfer CA (2007) Elasmobranchs of the Everglades national park. Bull Mar Sci 80:171–189
Acknowledgments
We thank Mote Marine Laboratory staff C. Simpfendorfer, B. Yeiser and A. Ubeda for their help with field efforts and data collection. We thank student volunteer interns M. Espinoza, K. Reiss, J. Price and A. Andyshak for field assistance. We would also like to express appreciation to C. Simpfendorfer, C. Wells, B. Blackwell, and M. Dachsteiner for their assistance with analysis, as well as anonymous reviewers for their manuscript comments. This research was funded in part by the South Florida Water Management District and the National Shark Research Consortium (NOAA Fisheries). L.A.O. was the recipient of the Mote Marine Laboratory and University of South Florida Graduate Fellowship in Elasmobranch Biology during the course of this work. Treatment of all animals in this study was conducted under ethical guidelines and approval for procedures was granted to M.R.H. by the MML IACUC Committee and to L.A.O. by the USF IACUC Committee under permit number 3020.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ortega, L.A., Heupel, M.R., Beynen, P.V. et al. Movement patterns and water quality preferences of juvenile bull sharks (Carcharhinus leucas) in a Florida estuary. Environ Biol Fish 84, 361–373 (2009). https://doi.org/10.1007/s10641-009-9442-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10641-009-9442-2