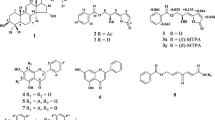
A new alkaloid, 6-hydroxy-β-carboline-1-carboxylic acid (1), together with six known alkaloids, including β-carboline-1-carboxylic acid (2), β-carboline-1-propanoic acid (3), 1-methoxycarbonyl-β-carboline (4), 3-methylcanthin-5,6-dione (5), 5-hydroxy-4-methoxycanthin-6-one (6), and 4,5-dimethoxycanthin-6-one (7), were isolated from the stem of Picrasma quassioides. Their structures were elucidated on the basis of spectroscopic data. Compounds 1–7 were screened for their cytotoxic activities against five cancer cell lines such as CT26.WT, K-562, SGC-7901, Hep G2, and A-549. Among the seven alkaloids tested, only compound 2 exhibited moderate inhibitory activities against K-562 and SGC-7901 cell lines.

Similar content being viewed by others
References
The State Pharmacopoeia Commission of P.R. China, Pharmacopoeia of the People’s Republic of China, Chemical Industry Press, Beijing, 2010, Vol. 1, p. 186.
W. H. Jiao, C. Y. Li, H. Gao, G. X. Zhou, P. Y. Sun, and X. S. Yao, Chin. Trad. Herb. Drugs, 38, 1419 (2007).
S. P. Yang and J. M. Yue, Helv. Chim. Acta, 87, 1591 (2004).
T. Ohmoto and K. Koike, Chem. Pharm. Bull., 30, 1204 (1982).
T. Ohmoto and K. Koike, Chem. Pharm. Bull., 32, 3579 (1984).
T. Ohmoto and K. Koike, Chem. Pharm. Bull., 33, 4901 (1985).
T. Ohmoto and K. Koike, Chem. Pharm. Bull., 33, 3847 (1985).
K. Koike and T. Ohmoto, Chem. Pharm. Bull., 34, 2090 (1986).
K. Koike and T. Ohmoto, Phytochemistry, 27, 3029 (1988).
W. H. Jiao, H. Gao, C. Y. Li, G. X. Zhou, S. Kitanaka, A. Ohmura, and X. S. Yao, Magn. Reson. Chem., 48, 490 (2010).
P. T. Chou, Y. I. Liu, G. R. Wu, M. Y. Shiao, and W. S. Yu, J. Phys. Chem., 105, 10674 (2001).
H. Hikino and T. Ohta, Phytochemistry, 14, 2473 (1975).
S. P. Yang and J. M. Yue, Helv. Chim. Acta, 87, 1591 (2004).
Y. Niimi, H. Hirota, and T. Tsuyuki, Chem. Pharm. Bull., 37, 57 (1989).
K. Yoshikawa, S. Sugawara, and S. Arihara, Phytochemistry, 40, 253 (1995).
M. Tsujimoto and H. Koyanagi, Bull. Chem. Soc. Jpn., 8, 161 (1933).
A. X. Zuo and G. X. Rao, Chem. Nat. Compd., 49, 980 (2013).
S. Fofana, R. Ziyaev, S. K. Diallo, M. Camara, and S. F. Aripova, Chem. Nat. Compd., 49, 587 (2013).
W. H. Jiao, H. Gao, F. Zhao, H.W. Lin, Y. M. Pan, G. X. Zhou, and X. S. Yao, Chem. Pharm. Bull., 59, 359 (2011).
T. Ohmoto, Y. I. Sung, K. Koike, and T. Nikaido, Shoyakugaku Zasshi, 39, 28 (1985).
Y. I. Sung, K. Koike, T. Nikaido, T. Ohmoto, and U. Sankawa, Chem. Pharm. Bull., 32, 1872 (1984).
J. Chen, X. H. Yan, J. H. Dong, P. Sang, X. Fang, Y. T. Di, Z. K. Zhang, and X. J. Hao, J. Agric. Food Chem., 57, 6590 (2009).
Y. Yin, S. I. Heo, K. S. Roh, and M. H. Wang, J. Plant. Biol., 52, 325 (2009).
Acknowledgment
The authors are grateful for financial support from the State Natural Science Funds Commission of the People’s Republic of China (Project No. u0732004), the Science and Technology Planning Project of Guangdong Province (No. 2009A030100014), and the Guangdong Province Universities and Colleges Pearl River Scholar Funded Scheme (2011).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Published in Khimiya Prirodnykh Soedinenii, No. 5, September–October, 2014, pp. 764–767.
Rights and permissions
About this article
Cite this article
Lai, ZQ., Liu, WH., Ip, SP. et al. Seven Alkaloids from Picrasma quassioides and Their Cytotoxic Activities. Chem Nat Compd 50, 884–888 (2014). https://doi.org/10.1007/s10600-014-1106-6
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10600-014-1106-6