Abstract
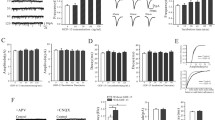
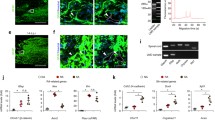
Acute injury of central nervous system (CNS) starts a cascade of morphological, molecular, and functional changes including formation of a fibrotic scar, expression of transforming growth factor beta 1 (TGF-β1), and expression of extracellular matrix proteins leading to arrested neurite outgrowth and failed regeneration. We assessed alteration of electrophysiological properties of cerebellar granule cells (CGCs) in two in vitro models of neuronal injury: (i) model of fibrotic scar created from coculture of meningeal fibroblasts and cerebral astrocytes with addition of TGF-β1; (ii) a simplified model based on administration of TGF-β1 to CGCs culture. Both models reproduced suppression of neurite outgrowth caused by neuronal injury, which was equally restored by chondroitinase ABC (ChABC), a key disruptor of fibrotic scar formation. Voltage-dependent calcium current was not affected in either injury model. However, intracellular calcium concentration could be altered as an expression of inositol trisphosphate receptor type 1 was suppressed by TGF-β1 and restored by ChABC. Voltage-dependent sodium current was significantly suppressed in CGCs cultured on a model of fibrotic scar and was only partly restored by ChABC. Administration of TGF-β1 significantly shifted current–voltage relation of sodium current toward more positive membrane potential without change to maximal current amplitude. Both transient and sustained potassium currents were significantly suppressed on a fibrotic scar and restored by ChABC to their control amplitudes. In contrast, TGF-β1 itself significantly upregulated transient and did not change sustained potassium current. Observed changes of voltage-dependent ion currents may contribute to known morphological and functional changes in injured CNS.






Similar content being viewed by others
References
Amico C, Marchetti C, Nobile M, Usai C (1995) Pharmacological types of calcium channels and their modulation by baclofen in cerebellar granules. J Neurosci 15(4):2839–2848
Anderson MA, Burda JE, Ren Y, Ao Y, O’Shea TM, Kawaguchi R, Coppola G, Khakh BS, Deming TJ, Sofroniew MV (2016) Astrocyte scar formation aids central nervous system axon regeneration. Nature 532(7598):195–200. doi:10.1038/nature17623
Berry M, Maxwell WL, Logan A, Mathewson A, McConnell P, Ashhurst DE, Thomas GH (1983) Deposition of scar tissue in the central nervous system. Acta Neurochir Suppl (Wien) 32:31–53
Brackenbury WJ, Calhoun JD, Chen C, Miyazaki H, Nukina N, Oyama F, Ranscht B, Isom LL (2010) Functional reciprocity between Na+ channel Nav1.6 and beta1 subunits in the coordinated regulation of excitability and neurite outgrowth. Proc Natl Acad Sci USA 107(5):2283–2288. doi:10.1073/pnas.0909434107
Cao Z, Cui Y, Busse E, Mehrotra S, Rainier JD, Murray TF (2014) Gambierol inhibition of voltage-gated potassium channels augments spontaneous Ca2+ oscillations in cerebrocortical neurons. J Pharmacol Exp Ther 350(3):615–623. doi:10.1124/jpet.114.215319
Carbonell AL, Boya J (1988) Ultrastructural study on meningeal regeneration and meningo-glial relationships after cerebral stab wound in the adult rat. Brain Res 439(1–2):337–344
Carlier E, Dargent B, De Waard M, Couraud F (2000) Na(+) channel regulation by calmodulin kinase II in rat cerebellar granule cells. Biochem Biophys Res Commun 274(2):394–399. doi:10.1006/bbrc.2000.3145
Carulli D, Laabs T, Geller HM, Fawcett JW (2005) Chondroitin sulfate proteoglycans in neural development and regeneration. Curr Opin Neurobiol 15(1):116–120. doi:10.1016/j.conb.2005.01.014
Cull-Candy SG, Marshall CG, Ogden D (1989) Voltage-activated membrane currents in rat cerebellar granule neurones. J Physiol 414:179–199
Davis TH, Chen C, Isom LL (2004) Sodium channel beta1 subunits promote neurite outgrowth in cerebellar granule neurons. J Biol Chem 279(49):51424–51432. doi:10.1074/jbc.M410830200
Fawcett JW, Asher RA (1999) The glial scar and central nervous system repair. Brain Res Bull 49(6):377–391
Fernandez-Klett F, Priller J (2014) The fibrotic scar in neurological disorders. Brain Pathol 24(4):404–413. doi:10.1111/bpa.12162
George J, Dravid SM, Prakash A, Xie J, Peterson J, Jabba SV, Baden DG, Murray TF (2009) Sodium channel activation augments NMDA receptor function and promotes neurite outgrowth in immature cerebrocortical neurons. J Neurosci 29(10):3288–3301. doi:10.1523/JNEUROSCI.6104-08.2009
Heath NC, Rizwan AP, Engbers JD, Anderson D, Zamponi GW, Turner RW (2014) The expression pattern of a Cav3-Kv4 complex differentially regulates spike output in cerebellar granule cells. J Neurosci 34(26):8800–8812. doi:10.1523/JNEUROSCI.0981-14.2014
Ishikawa K, Tanaka M, Black JA, Waxman SG (1999) Changes in expression of voltage-gated potassium channels in dorsal root ganglion neurons following axotomy. Muscle Nerve 22(4):502–507. doi:10.1002/(Sici)1097-4598(199904)22:4<502:Aid-Mus12>3.0.Co;2-K
Jabba SV, Prakash A, Dravid SM, Gerwick WH, Murray TF (2010) Antillatoxin, a novel lipopeptide, enhances neurite outgrowth in immature cerebrocortical neurons through activation of voltage-gated sodium channels. J Pharmacol Exp Ther 332(3):698–709. doi:10.1124/jpet.109.161802
Jalonen T, Johansson S, Holopainen I, Oja SS, Arhem P (1990) Single-channel and whole-cell currents in rat cerebellar granule cells. Brain Res 535(1):33–38
Jaskova K, Pavlovicova M, Jurkovicova D (2012) Calcium transporters and their role in the development of neuronal disease and neuronal damage. Gen Physiol Biophys 31(4):375–382. doi:10.4149/gpb_2012_053
Jaskova K, Pavlovicova M, Cagalinec M, Lacinova L, Jurkovicova D (2014) TGFbeta1 downregulates neurite outgrowth, expression of Ca2 + transporters, and mitochondrial dynamics of in vitro cerebellar granule cells. NeuroReport 25(5):340–346. doi:10.1097/WNR.0000000000000106
Jiao S, Liu Z, Ren WH, Ding Y, Zhang YQ, Zhang ZH, Mei YA (2007) cAMP/protein kinase A signalling pathway protects against neuronal apoptosis and is associated with modulation of Kv2.1 in cerebellar granule cells. J Neurochem 100(4):979–991. doi:10.1111/j.1471-4159.2006.04261.x
Jurkovicova D, Kopacek J, Stefanik P, Kubovcakova L, Zahradnikova A Jr, Zahradnikova A, Pastorekova S, Krizanova O (2007) Hypoxia modulates gene expression of IP3 receptors in rodent cerebellum. Pflugers Arch 454(3):415–425. doi:10.1007/s00424-007-0214-6
Karmazinova M, Lacinova L (2010) Measurement of cellular excitability by whole cell patch clamp technique. Physiol Res 59(Suppl 1):S1–S7
Kawano H, Kimura-Kuroda J, Komuta Y, Yoshioka N, Li HP, Kawamura K, Li Y, Raisman G (2012) Role of the lesion scar in the response to damage and repair of the central nervous system. Cell Tissue Res 349(1):169–180. doi:10.1007/s00441-012-1336-5
Kimura-Kuroda J, Teng X, Komuta Y, Yoshioka N, Sango K, Kawamura K, Raisman G, Kawano H (2010) An in vitro model of the inhibition of axon growth in the lesion scar formed after central nervous system injury. Mol Cell Neurosci 43(2):177–187. doi:10.1016/j.mcn.2009.10.008
Komuta Y, Teng X, Yanagisawa H, Sango K, Kawamura K, Kawano H (2010) Expression of transforming growth factor-beta receptors in meningeal fibroblasts of the injured mouse brain. Cell Mol Neurobiol 30(1):101–111. doi:10.1007/s10571-009-9435-x
Li HP, Homma A, Sango K, Kawamura K, Raisman G, Kawano H (2007) Regeneration of nigrostriatal dopaminergic axons by degradation of chondroitin sulfate is accompanied by elimination of the fibrotic scar and glia limitans in the lesion site. J Neurosci Res 85(3):536–547. doi:10.1002/jnr.21141
Li HP, Komuta Y, Kimura-Kuroda J, van Kuppevelt TH, Kawano H (2013) Roles of chondroitin sulfate and dermatan sulfate in the formation of a lesion scar and axonal regeneration after traumatic injury of the mouse brain. J Neurotrauma 30(5):413–425. doi:10.1089/neu.2012.2513
Logan A, Berry M, Gonzalez AM, Frautschy SA, Sporn MB, Baird A (1994) Effects of transforming growth factor beta 1 on scar production in the injured central nervous system of the rat. Eur J Neurosci 6(3):355–363
Ma SH, Li B, Huang HW, Peng YP, Qiu YH (2012) Interleukin-6 inhibits L-type calcium channel activity of cultured cerebellar granule neurons. J Physiol Sci 62(5):385–392. doi:10.1007/s12576-012-0215-x
Mathie A, Clarke CE, Ranatunga KM, Veale EL (2003) What are the roles of the many different types of potassium channel expressed in cerebellar granule cells? Cerebellum 2(1):11–25. doi:10.1080/14734220310015593
Mattson MP (2007) Calcium and neurodegeneration. Aging Cell 6(3):337–350. doi:10.1111/j.1474-9726.2007.00275.x
Moreira-Lobo DC, Cruz JS, Silva FR, Ribeiro FM, Kushmerick C, Oliveira FA (2016) Thiamine deficiency increases Ca current and Ca1.2 L-type Ca channel levels in cerebellum granular neurons. Cell Mol Neurobiol. doi:10.1007/s10571-016-0378-8
Morgenstern DA, Asher RA, Fawcett JW (2002) Chondroitin sulphate proteoglycans in the CNS injury response. Prog Brain Res 137:313–332
Murali SS, Napier IA, Mohammadi SA, Alewood PF, Lewis RJ, Christie MJ (2015) High-voltage-activated calcium current subtypes in mouse DRG neurons adapt in a subpopulation-specific manner after nerve injury. J Neurophysiol 113(5):1511–1519. doi:10.1152/jn.00608.2014
Nichols J, Perez R, Wu C, Adelson PD, Anderson T (2015) Traumatic brain injury induces rapid enhancement of cortical excitability in juvenile rats. CNS Neurosci Ther 21(2):193–203. doi:10.1111/cns.12351
Rossi P, D’Angelo E, Magistretti J, Toselli M, Taglietti V (1994) Age-dependent expression of high-voltage activated calcium currents during cerebellar granule cell development in situ. Pflugers Arch 429(1):107–116
Schaller KL, Caldwell JH (2003) Expression and distribution of voltage-gated sodium channels in the cerebellum. Cerebellum 2(1):2–9. doi:10.1080/14734220309424
Shearer MC, Fawcett JW (2001) The astrocyte/meningeal cell interface—a barrier to successful nerve regeneration? Cell Tissue Res 305(2):267–273
Shearer MC, Niclou SP, Brown D, Asher RA, Holtmaat AJ, Levine JM, Verhaagen J, Fawcett JW (2003) The astrocyte/meningeal cell interface is a barrier to neurite outgrowth which can be overcome by manipulation of inhibitory molecules or axonal signalling pathways. Mol Cell Neurosci 24(4):913–925
Shibata R, Nakahira K, Shibasaki K, Wakazono Y, Imoto K, Ikenaka K (2000) A-type K+ current mediated by the Kv4 channel regulates the generation of action potential in developing cerebellar granule cells. J Neurosci 20(11):4145–4155
Stewart RR, Bossu JL, Muzet M, Dupont JL, Feltz A (1995) Voltage-activated ionic currents in differentiating rat cerebellar granule neurons cultured from the external germinal layer. J Neurobiol 28(4):419–432. doi:10.1002/neu.480280403
Struckhoff G (1995) Cocultures of meningeal and astrocytic cells—a model for the formation of the glial-limiting membrane. Int J Dev Neurosci 13(6):595–606
Susarla BT, Laing ED, Yu P, Katagiri Y, Geller HM, Symes AJ (2011) Smad proteins differentially regulate transforming growth factor-beta-mediated induction of chondroitin sulfate proteoglycans. J Neurochem 119(4):868–878. doi:10.1111/j.1471-4159.2011.07470.x
Toth AB, Shum AK, Prakriya M (2016) Regulation of neurogenesis by calcium signaling. Cell Calcium 59(2–3):124–134. doi:10.1016/j.ceca.2016.02.011
Viviani B, Gardoni F, Marinovich M (2007) Cytokines and neuronal ion channels in health and disease. Int Rev Neurobiol 82:247–263. doi:10.1016/S0074-7742(07)82013-7
Wanner IB, Deik A, Torres M, Rosendahl A, Neary JT, Lemmon VP, Bixby JL (2008) A new in vitro model of the glial scar inhibits axon growth. Glia 56(15):1691–1709. doi:10.1002/glia.20721
Zhuang JL, Wang CY, Zhou MH, Duan KZ, Mei YA (2012) TGF-beta 1 Enhances Kv2.1 potassium channel protein expression and promotes maturation of cerebellar granule neurons. J Cell Physiol 227(1):297–307. doi:10.1002/jcp.22735
Acknowledgments
This work was supported by the Slovak Research and Development Agency under the contract No. APVV-0212-10.
Authors Contribution
Katarína Ondáčová did the experiments, evaluated data, and participated in writing manuscript. Dana Jurkovičová designed and supervised experiments, and participated in writing manuscript. Ľubica Lacinová designed and supervised experiments, participated in data evaluation, and participated in writing manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Authors declare no conflict of interest and no financial interest in the publication of this manuscript
Rights and permissions
About this article
Cite this article
Ondáčová, K., Jurkovičová, D. & Lacinová, Ľ. Altered Sodium and Potassium, but not Calcium Currents in Cerebellar Granule Cells in an In Vitro Model of Neuronal Injury. Cell Mol Neurobiol 37, 771–782 (2017). https://doi.org/10.1007/s10571-016-0416-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10571-016-0416-6