Abstract
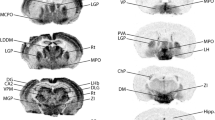
Among all K2P channels, TASK-3 shows the most widespread expression in rat brain, regulating neuronal excitability and transmitter release. Using a recently purified and characterized polyclonal monospecific antibody against TASK-3, the entire rat brain was immunocytochemically analyzed for expression of TASK-3 protein. Besides its well-known strong expression in motoneurons and monoaminergic and cholinergic neurons, TASK-3 expression was found in most neurons throughout the brain. However, it was not detected in certain neuronal populations, and neuropil staining was restricted to few areas. Also, it was absent in adult glial cells. In hypothalamic areas, TASK-3 was particularly strongly expressed in the supraoptic and suprachiasmatic nuclei, whereas other hypothalamic nuclei showed lower protein levels. Immunostaining of hippocampal CA1 and CA3 pyramidal neurons showed strongest expression, together with clear staining of CA3 mossy fibers and marked staining also in the dentate gyrus granule cells. In neocortical areas, most neurons expressed TASK-3 with a somatodendritic localization, most obvious in layer V pyramidal neurons. In the cerebellum, TASK-3 protein was found mainly in neurons and neuropil of the granular cell layer, whereas Purkinje cells were only faintly positive. Particularly weak expression was demonstrated in the forebrain. This report provides a comprehensive overview of TASK-3 protein expression in the rat brain.






Similar content being viewed by others
References
Bayliss DA, Talley EM, Sirois JE, Lei Q (2001) TASK-1 is a highly modulated pH-sensitive ‘leak’ K(+) channel expressed in brainstem respiratory neurons. Respir Physiol 129:159–174
Berg AP, Bayliss DA (2007) Striatal cholinergic interneurons express a receptor-insensitive homomeric TASK-3-like background K+ current. J Neurophysiol 97:1546–1552
Berg AP, Talley EM, Manger JP, Bayliss DA (2004) Motoneurons express heteromeric TWIK-related acid-sensitive K+ (TASK) channels containing TASK-1 (KCNK3) and TASK-3 (KCNK9) subunits. J Neurosci 24:6693–6702
Callahan R, Labunskiy DA, Logvinova A, Abdallah M, Liu C, Cotten JF, Yost CS (2004) Immunolocalization of TASK-3 (KCNK9) to a subset of cortical neurons in the rat CNS. Biochem Biophys Res Commun 319:525–530
Czirjak G, Fischer T, Spat A, Lesage F, Enyedi P (2000) TASK (TWIK-related acid-sensitive K+ channel) is expressed in glomerulosa cells of rat adrenal cortex and inhibited by angiotensin II. Mol Endocrinol 14:863–874
Gotter AL, Santarelli VP, Doran SM, Tannenbaum PL, Kraus RL, Rosahl TW, Meziane H, Montial M, Reiss DR, Wessner K, McCampbell A, Stevens J, Brunner JI, Fox SV, Uebele VN, Bayliss DA, Winrow CJ, Renger JJ (2011) TASK-3 as a potential antidepressant target. Brain Res 1416:69–79
Hopwood SE, Trapp S (2005) TASK-like K+ channels mediate effects of 5-HT and extracellular pH in rat dorsal vagal neurones in vitro. J Physiol 568:145–154
Kananura C, Sander T, Rajan S, Preisig-Muller R, Grzeschik KH, Daut J, Derst C, Steinlein OK (2002) Tandem pore domain K(+)-channel TASK-3 (KCNK9) and idiopathic absence epilepsies. Am J Med Genet 114:227–229
Karschin C, Wischmeyer E, Preisig-Muller R, Rajan S, Derst C, Grzeschik KH, Daut J, Karschin A (2001) Expression pattern in brain of TASK-1, TASK-3, and a tandem pore domain K(+) channel subunit, TASK-5, associated with the central auditory nervous system. Mol Cell Neurosci 18:632–648
Kim JE, Kwak SE, Kang TC (2009) Upregulated TWIK-related acid-sensitive K+ channel-2 in neurons and perivascular astrocytes in the hippocampus of experimental temporal lobe epilepsy. Epilepsia 50:654–663
Kim JE, Yeo SI, Ryu HJ, Chung CK, Kim MJ, Kang TC (2011) Changes in TWIK-related acid sensitive K+ -1 and -3 channel expressions from neurons to glia in the hippocampus of temporal lobe epilepsy patients and experimental animal model. Neurochem Res 36:2155–2168
Linden AM, Sandu C, Aller MI, Vekovischeva OY, Rosenberg PH, Wisden W, Korpi ER (2007) TASK-3 knockout mice exhibit exaggerated nocturnal activity, impairments in cognitive functions, and reduced sensitivity to inhalation anesthetics. J Pharmacol Exp Ther 323:924–934
Link W, Konietzko U, Kauselmann G, Krug M, Schwanke B, Frey U, Kuhl D (1995) Somatodendritic expression of an immediate early gene is regulated by synaptic activity. Proc Natl Acad Sci USA 92:5734–5738
Marinc C, Preisig-Muller R, Prüss H, Derst C, Veh RW (2011) Immunocytochemical localization of TASK-3 (K(2P)9.1) channels in monoaminergic and cholinergic neurons. Cell Mol Neurobiol 31:323–335
Marinc C, Prüss H, Derst C, Veh RW (2012) Immunocytochemical localization of TASK-3 channels in rat motor neurons. Cell Mol Neurobiol 32:309–318
Meadows HJ, Randall AD (2001) Functional characterisation of human TASK-3, an acid-sensitive two-pore domain potassium channel. Neuropharmacology 40:551–559
Meuth SG, Budde T, Kanyshkova T, Broicher T, Munsch T, Pape HC (2003) Contribution of TWIK-related acid-sensitive K+ channel 1 (TASK1) and TASK3 channels to the control of activity modes in thalamocortical neurons. J Neurosci 23:6460–6469
Millar JA, Barratt L, Southan AP, Page KM, Fyffe RE, Robertson B, Mathie A (2000) A functional role for the two-pore domain potassium channel TASK-1 in cerebellar granule neurons. Proc Natl Acad Sci USA 97:3614–3618
Patel AJ, Honore E (2001) Properties and modulation of mammalian 2P domain K+ channels. Trends Neurosci 24:339–346
Patel AJ, Honore E, Lesage F, Fink M, Romey G, Lazdunski M (1999) Inhalational anesthetics activate two-pore-domain background K+ channels. Nat Neurosci 2:422–426
Paxinos G, Watson C (1998) The rat brain in stereotaxic coordinates, 4th edn. Academic Press, San Diego
Pradidarcheep W, Labruyere WT, Dabhoiwala NF, Lamers WH (2008) Lack of specificity of commercially available antisera: better specifications needed. J Histochem Cytochem 56:1099–1111
Prüss H, Dewes M, Derst C, Fernandez-Klett F, Veh RW, Priller J (2011) Potassium channel expression in adult murine neural progenitor cells. Neuroscience 180:19–29
Rajan S, Wischmeyer E, Karschin C, Preisig-Muller R, Grzeschik KH, Daut J, Karschin A, Derst C (2001) THIK-1 and THIK-2, a novel subfamily of tandem pore domain K+ channels. J Biol Chem 276:7302–7311
Rajan S, Preisig-Muller R, Wischmeyer E, Nehring R, Hanley PJ, Renigunta V, Musset B, Schlichthorl G, Derst C, Karschin A, Daut J (2002) Interaction with 14-3-3 proteins promotes functional expression of the potassium channels TASK-1 and TASK-3. J Physiol 545:13–26
Renigunta V, Yuan H, Zuzarte M, Rinne S, Koch A, Wischmeyer E, Schlichthorl G, Gao Y, Karschin A, Jacob R, Schwappach B, Daut J, Preisig-Muller R (2006) The retention factor p11 confers an endoplasmic reticulum-localization signal to the potassium channel TASK-1. Traffic 7:168–181
Rusznak Z, Pocsai K, Kovacs I, Por A, Pal B, Biro T, Szucs G (2004) Differential distribution of TASK-1, TASK-2 and TASK-3 immunoreactivities in the rat and human cerebellum. Cell Mol Life Sci 61:1532–1542
Saper CB (2009) A guide to the perplexed on the specificity of antibodies. J Histochem Cytochem 57:1–5
Simat M, Parpan F, Fritschy JM (2007) Heterogeneity of glycinergic and gabaergic interneurons in the granule cell layer of mouse cerebellum. J Comp Neurol 500:71–83
Skatchkov SN, Eaton MJ, Shuba YM, Kucheryavykh YV, Derst C, Veh RW, Wurm A, Iandiev I, Pannicke T, Bringmann A, Reichenbach A (2006) Tandem-pore domain potassium channels are functionally expressed in retinal (Muller) glial cells. Glia 53:266–276
Talley EM, Bayliss DA (2002) Modulation of TASK-1 (Kcnk3) and TASK-3 (Kcnk9) potassium channels: volatile anesthetics and neurotransmitters share a molecular site of action. J Biol Chem 277:17733–17742
Talley EM, Lei Q, Sirois JE, Bayliss DA (2000) TASK-1, a two-pore domain K+ channel, is modulated by multiple neurotransmitters in motoneurons. Neuron 25:399–410
Talley EM, Solorzano G, Lei Q, Kim D, Bayliss DA (2001) Cns distribution of members of the two-pore-domain (KCNK) potassium channel family. J Neurosci 21:7491–7505
Torborg CL, Berg AP, Jeffries BW, Bayliss DA, McBain CJ (2006) TASK-like conductances are present within hippocampal CA1 stratum oriens interneuron subpopulations. J Neurosci 26:7362–7367
Veh RW, Lichtinghagen R, Sewing S, Wunder F, Grumbach IM, Pongs O (1995) Immunohistochemical localization of five members of the Kv1 channel subunits: contrasting subcellular locations and neuron-specific co-localizations in rat brain. Eur J Neurosci 7:2189–2205
Conflict of interest
All authors declare that there are no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Marinc, C., Derst, C., Prüss, H. et al. Immunocytochemical Localization of TASK-3 Protein (K2P9.1) in the Rat Brain. Cell Mol Neurobiol 34, 61–70 (2014). https://doi.org/10.1007/s10571-013-9987-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10571-013-9987-7