Abstract
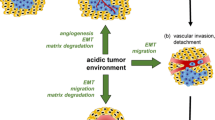
Tumors are ecosystems which develop from stem cells endowed with unlimited self-renewal capability and genetic instability, under the effects of mutagenesis and natural selection imposed by environmental changes. Abnormal vascularization, reduced lymphatic network, uncontrolled cell growth frequently associated with hypoxia, and extracellular accumulation of glucose metabolites even in the presence of an adequate oxygen level are all factors contributing to reduce pH in the extracellular space of tumors. Evidence is accumulating that acidity is associated with a poor prognosis and participates actively to tumor progression. This review addresses some of the most experimental evidences providing that acidity of tumor environment facilitates local invasiveness and metastatic dissemination, independently from hypoxia, with which acidity is often but not always associated. Clinical investigations have also shown that tumors with acidic environment are associated with resistance to chemotherapy and radiation-induced apoptosis, suppression of cytotoxic lymphocytes, and natural killer cells tumoricidal activity. Therefore, new technologies for functional and molecular imaging as well as strategies directed to target low extracellular pH and low pH-adapted tumor cells might represent important issues in oncology.

Similar content being viewed by others
References
Nicolson, G. L. (1984). Tumor progression, oncogenes and the evolution of metastatic phenotypic diversity. Clinical and Experimental Metastasis, 2, 85–105.
Miller, F. R., & Heppner, G. H. (1990). Cellular interactions in metastasis. Cancer and Metastasis Reviews, 9, 21–34.
Witz, I. P. (2008). Tumor-microenvironment interactions: dangerous liaisons. Advances in Cancer Research, 100, 203–229.
Nguyen, D. X., Bos, P. D., & Massague, J. (2009). Metastasis: from dissemination to organ-specific colonization. Nature Reviews Cancer, 9, 274–284.
Joyce, J. A., & Pollard, J. W. (2009). Microenvironmental regulation of metastasis. Nature Reviews Cancer, 9, 239–252.
Aguirre-Ghiso, J. A. (2007). Models, mechanisms and clinical evidence for cancer dormancy. Nature Reviews Cancer, 7, 834–846.
Rubin, H. (2008). Contact interactions between cells that suppress neoplastic development: can they also explain metastatic dormancy? Advances in Cancer Research, 100, 159–202.
Hanahan, D., & Weinberg, R. A. (2011). Hallmarks of cancer: the next generation. Cell, 144, 646–674.
Tammela, T., & Alitalo, K. (2010). Lymphangiogenesis: molecular mechanisms and future promise. Cell, 140, 460–476.
Karkkainen, M. J., Haiko, P., Sainio, K., Partanen, J., Taipale, J., Petrova, T. V., et al. (2004). Vascular endothelial growth factor C is required for sprouting of the first lymphatic vessels from embryonic veins. Nature Immunology, 5, 74–80.
Fukumura, D., & Jain, R. K. (2007). Tumor microenvironment abnormalities: causes, consequences, and strategies to normalize. Journal of Cellular Biochemistry, 101, 937–949.
Racker, E. (1974). History of the Pasteur effect and its pathobiology. Molecular and Cellular Biochemistry, 5, 17–23.
Gatenby, R. A., & Gillies, R. J. (2004). Why do cancers have high aerobic glycolysis? Nature Reviews Cancer, 4, 891–899.
Vander Heiden, M. G., Cantley, L. C., & Thompson, C. B. (2009). Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science, 324, 1029–1033.
Klement, R. J., & Kämmerer, U. (2011). Is there a role for carbohydrate restriction in the treatment and prevention of cancer? Nutrition & Metabolism (London), 8, 75.
Denko, N. C. (2008). Hypoxia, HIF1 and glucose metabolism in the solid tumour. Nature Reviews Cancer, 8, 705–713.
DeBerardinis, R. J. (2008). Is cancer a disease of abnormal cellular metabolism? New angles on an old idea. Genetics in Medicine, 10, 767–777.
Hirschhaeuser, F., Sattler, U. G., & Mueller-Klieser, W. (2011). Lactate: a metabolic key player in cancer. Cancer Research, 71(22), 6921–6925.
Lu, H., Forbes, R. A., & Verma, A. (2002). Hypoxia-inducible factor 1 activation by aerobic glycolysis implicates the Warburg effect in carcinogenesis. Journal of Biological Chemistry, 277, 23111–23115.
Ebert, B. L., Firth, J. D., & Ratcliffe, P. J. (1995). Hypoxia and mitochondrial inhibitors regulate expression of glucose transporter-1 via distinct Cis-acting sequences. Journal of Biological Chemistry, 270(49), 29083–29089.
Kim, J. W., Tchernyshyov, I., Semenza, G. L., & Dang, C. V. (2006). HIF-1-mediated expression of pyruvate dehydrogenase kinase: a metabolic switchrequired for cellular adaptation to hypoxia. Cell Metabolism, 3, 177–185.
Semenza, G. L., Jiang, B. H., Leung, S. W., Passantino, R., Concordet, J. P., Maire, P., et al. (1996). Hypoxia response elements in the aldolase A, enolase 1, and lactate dehydrogenase A gene promoters contain essential binding sites for hypoxia-inducible factor 1. Journal of Biological Chemistry, 271(51), 32529–32537.
Cairns, R. A., Harris, I., McCracken, S., & Mak, T. W. (2011). Cancer cell metabolism. Cold Spring Harbor Symposia on Quantitative Biology, 76, 299–311.
Sonveaux, P., Vegran, F., Schroeder, T., Wergin, M. C., Verrax, J., Rabbani, Z. N., et al. (2008). Targeting lactate-fueled respiration selectively kills hypoxic tumor cells in mice. Journal of Clinical Investigation, 118, 3930–3942.
Feron, O. (2009). Pyruvate into lactate and back: from the Warburg effect to symbiotic energy fuel exchange in cancer cells. Radiotherapy and Oncology, 92, 329–333.
Whitaker-Menezes, D., Martinez-Outschoorn, U. E., Lin, Z., Ertel, A., Flomenberg, N., Witkiewicz, A. K., et al. (2011). Evidence for a stromal-epithelial ‘lactate shuttle’ in human tumors: MCT4 is a marker of oxidative stress in cancer-associated fibroblasts. Cell Cycle, 10, 1772–1783.
Brooks, G. A. (2009). Cell-cell and intracellular lactate shuttles. Journal of Physiology, 587, 5591–5600.
Gladden, L. B. (2004). Lactate metabolism: a new paradigm for the third millennium. Journal of Physiology, 558, 5–30.
Guido, C., Whitaker-Menezes, D., Capparelli, C., Balliet, R., Lin, Z., Pestell, R. G., et al. (2012). Metabolic reprogramming of cancer-associated fibroblasts by TGF-β drives tumor growth: connecting TGF-β signaling with “Warburg-like” cancer metabolism and l-lactate production. Cell Cycle, 11(16), 3019–3035.
Fiaschi, T., Marini, A., Giannoni, E., Taddei, M. L., Gandellini, P., De Donatis, A., et al. (2012). Reciprocal metabolic reprogramming through lactate shuttle coordinately influences tumor-stroma interplay. Cancer Research, 72, 5130–5140.
Roos, A., & Boron, W. F. (1981). Intracellular pH. Physiological Reviews, 61, 296–434.
Parks, S. K., Chiche, J., & Pouysségur, J. (2013). Disrupting proton dynamics and energy metabolism for cancer therapy. Nature Reviews Cancer, 13(9), 611–623.
Calorini, L., Peppicelli, S., & Bianchini, F. (2012). Extracellular acidity as favouring factor of tumor progression and metastatic dissemination. Experimental Oncology, 34(2), 79–84.
Walenta, S., Wetterling, M., Lehrke, M., Schwickert, G., Sundfør, K., Rofstad, E. K., et al. (2000). High lactate levels predict likelihood of metastases, tumor recurrence, and restricted patient survival in human cervical cancers. Cancer Research, 60, 916–921.
Morita, T., Nagaki, T., Fukuda, I., & Okumura, K. (1992). Clastogenicity of low pH to various cultured mammalian cells. Mutation Research, 268, 297–305.
Raghunand, N., Mahoney, B., van Sluis, R., Baggett, B., & Gillies, R. J. (2001). Acute metabolic alkalosis enhances response of C3H mouse mammary tumors to the weak base mitoxantrone. Neoplasia, 3, 227–235.
Rottinger, E. M., & Mendonca, M. (1982). Radioresistance secondary to low pH in human glial cells and Chinese hamster ovary cells. International Journal of Radiation Oncology, Biology, and Physics, 8, 1309–1314.
Webb, B. A., Chimenti, M., Jacobson, M. P., & Barber, D. L. (2011). Dysregulated pH: a perfect storm for cancer progression. Nature Reviews Cancer, 11, 671–677.
Provent, P., Benito, M., Hiba, B., Farion, R., López-Larrubia, P., Ballesteros, P., et al. (2007). Serial in vivo spectroscopic nuclear magnetic resonance imaging of lactate and extracellular pH in rat gliomas shows redistribution of protons away from sites of glycolysis. Cancer Research, 67, 7638–7645.
Pouyssegur, J., Dayan, F., & Mazure, N. M. (2006). Hypoxia signalling in cancer and approaches to enforce tumour regression. Nature, 441, 437–443.
Helmlinger, G., Yuan, F., Dellian, M., & Jain, R. K. (1997). Interstitial pH and pO2 gradients in solid tumors in vivo: high-resolution measurements reveal a lack of correlation. Nature Medicine, 3, 177–182.
Ridley, A. J., Schwartz, M. A., Burridge, K., Firtel, R. A., Ginsberg, M. H., Borisy, G., et al. (2003). Cell migration: integrating signals from front to back. Science, 302, 1704–1709.
Pope, B. J., Zierler-Gould, K. M., Kühne, R., Weeds, A. G., & Ball, L. J. (2004). Solution structure of human cofilin: actin binding, pH sensitivity, and relationship to actin-depolymerizing factor. Journal of Biological Chemistry, 279(6), 4840–4848.
McLachlan, G. D., Cahill, S. M., Girvin, M. E., & Almo, S. C. (2007). Acid-induced equilibrium folding intermediate of human platelet profiling. Biochemistry, 46, 6931–6943.
Moseley, J. B., Okada, K., Balcer, H. I., Kovar, D. R., Pollard, T. D., & Goode, B. L. (2006). Twinfilin is an actin-filament-severing protein and promotes rapid turnover of actin structures in vivo. Journal of Cell Science, 119, 1547–1557.
Grey, M. J., Tang, Y., Alexov, E., McKnight, C. J., Raleigh, D. P., & Palmer, A. G., III. (2006). Characterizing a partially folded intermediate of the villin headpiece domain under non-denaturing conditions: contribution of His41 to the pHdependent stability of the N-terminal subdomain. Journal of Molecular Biology, 355, 1078–1094.
Srivastava, J., Barreiro, G., Groscurth, S., Gingras, A. R., Goult, B. T., Critchley, D. R., et al. (2008). Structural model and functional significance of pH-dependent talin-actin binding for focal adhesion remodeling. Proceedings of the National Academy of Sciences of the United States of America, 105, 14436–14441.
Frantz, C., Karydis, A., Nalbant, P., Hahn, K. M., & Barber, D. L. (2007). Positive feedback between Cdc42 activity and H+ efflux by the Na–H exchanger NHE1 for polarity of migrating cells. The Journal of Cell Biology, 179, 403–410.
Stock, C., Cardone, R. A., Busco, G., Krähling, H., Schwab, A., & Reshkin, S. J. (2008). Protons extruded by NHE1: digestive or glue? European Journal of Cell Biology, 87, 591–599.
Paradise, R. K., Lauffenburger, D. A., & Van Vliet, K. J. (2011). Acidic extracellular pH promotes activation of integrin αvβ3. PLoS ONE, 6, e15746.
Brisson, L., Reshkin, S. J., Goré, J., & Roger, S. (2012). pH regulators in invadosomal functioning: proton delivery for matrix tasting. European Journal of Cell Biology, 91, 847–860.
Lucien, F., Brochu-Gaudreau, K., Arsenault, D., Harper, K., & Dubois, C. M. (2011). Hypoxia-induced invadopodia formation involves activation of NHE-1 by the p90 ribosomal S6 kinase (p90RSK). PLoS One, 6, e28851.
Attanasio, F., Caldieri, G., Giacchetti, G., van Horssen, R., Wieringa, B., & Buccione, R. (2011). Novel invadopodia components revealed by differential proteomic analysis. European Journal of Cell Biology, 90, 115–127.
Rozhin, J., Sameni, M., Ziegler, G., & Sloane, F. B. (1994). Pericellular pH affects distribution and secretion of cathepsin B in malignant cells. Cancer Research, 54, 6517–6525.
Webb, S. D., Sherratt, J. A., & Fish, R. G. (1999). Alterations in proteolytic activity at low pH and its association with invasion: a theoretical model. Clinical and Experimental Metastasis, 17, 397–407.
Goretzki, L., Schmitt, M., Mann, K., Calvete, J., Chucholowski, N., Kramer, M., et al. (1992). Effective activation of the proenzyme form of the urokinase-type plasminogen activator (pro-uPA) by the cysteine protease cathepsin L. FEBS Letters, 297, 112–118.
Mignatti, P., & Rifkin, D. B. (1996). Plasminogen activators and matrix metalloproteinases in angiogenesis. Enzyme and Protein, 49, 117–137.
Lyons, R. M., Keski-Oja, J., & Moses, H. L. (1988). Proteolytic activation of latent transforming growth factor-beta from fibroblast-conditioned medium. Journal of Cell Biology, 106, 1659–1665.
Ellis, V., Pyke, C., Eriksen, J., Solberg, H., & Danø, K. (1992). The urokinase receptor: involvement in cell surface proteolysis and cancer invasion. Annals of the New York Academy of Sciences, 667, 13–31.
Nagase, H., & Woessner, J. F. (1999). Matrix metalloproteinases. Journal of Biological Chemistry, 274, 21491–21494.
Vihinen, P., & Kähäri, V. M. (2002). Matrix metalloproteinases in cancer: prognostic markers and therapeutic targets. International Journal of Cancer, 99(2), 157–166.
Itoh, T., Tanioka, M., Matsuda, H., Nishimoto, H., Yoshioka, T., Suzuki, R., et al. (1999). Experimental metastasis is suppressed in MMP-9-deficient mice. Clinical and Experimental Metastasis, 17(2), 177–181.
Itoh, T., Tanioka, M., Yoshida, H., Yoshioka, T., Nishimoto, H., & Itohara, S. (1998). Reduced angiogenesis and tumor progression in gelatinase A-deficient mice. Cancer Research, 58(5), 1048–1051.
Kato, Y., Nakayama, Y., Umeda, M., & Miyazaki, K. (1992). Induction of 103-kDa gelatinase/type IV collagenase by acidic culture conditions in mouse metastatic melanoma cell lines. Journal of Biological Chemistry, 267(16), 11424–11430.
Toyoshima, M., & Nakajima, M. (1999). Human heparanase. Purification, characterization, cloning, and expression. Journal of Biological Chemistry, 274, 24153–24160.
Shi, Q., Le, X., Wang, B., Abbruzzese, J. L., Xiong, Q., He, Y., et al. (2001). Regulation of vascular endothelial growth factor expression by acidosis in human cancer cells. Oncogene, 20, 3751–3756.
Fukumura, D., Xu, L., Chen, Y., Gohongi, T., Seed, B., & Jain, R. K. (2000). Hypoxia and acidosis independently up-regulate vascular endothelial growth factor transcription in brain tumors in vivo. Cancer Research, 61, 6020–6024.
Xu, L., & Fidler, I. J. (2000). Acidic pH-induced elevation in interleukin 8 expression by human ovarian carcinoma cells. Cancer Research, 60(16), 4610–4616.
Peppicelli, S., Bianchini, F., Contena, C., Tombaccini, D., & Calorini, L. (2013). Acidic pH via NF-κB favours VEGF-C expression in human melanoma cells. Clinical and Experimental Metastasis, 30(8), 957–967.
Su, J. L., Yang, P. C., Shih, J. Y., Yang, C. Y., Wei, L. H., Hsieh, C. Y., et al. (2006). The VEGF-C/Flt-4 axis promotes invasion and metastasis of cancer cells. Cancer Cell, 9, 209–223.
Radisky, D. C. (2005). Epithelial-mesenchymal transition. Journal of Cell Science, 118(Pt 19), 4325–4326.
Kalluri, R., & Neilson, E. G. (2003). Epithelial-mesenchymal transition and its implications for fibrosis. The Journal of Clinical Investigation, 112(12), 1776–1784.
Kalluri, R. (2009). EMT: when epithelial cells decide to become mesenchymal-like cells. The Journal of Clinical Investigation, 119(6), 1417–1419.
Peppicelli, S., Bianchini, F., Torre, E., Calorini, L. (2014). Contribution of acidic melanoma cells undergoing epithelial-to-mesenchymal transition to aggressiveness of non-acidic melanoma cells. Clinical and Experimental Metastasis.
Xue, L., & Lucocq, J. M. (1997). Low extracellular pH induces activation of ERK 2, JNK, and p38 in A431 and Swiss 3 T3 cells. Biochemical and Biophysical Research Communications, 241(2), 236–242.
Sarosi, G. A., Jr., Jaiswal, K., Herndon, E., Lopez-Guzman, C., Spechler, S. J., & Souza, R. F. (2005). Acid increases MAPK-mediated proliferation in Barrett’s esophageal adenocarcinoma cells via intracellular acidification through a Cl-/HCO3- exchanger. American Journal of Physiology - Gastrointestinal and Liver Physiology, 289(6), G991–G997.
Kumar, S., Reusch, H. P., & Ladilov, Y. (2008). Acidic pre-conditioning suppresses apoptosis and increases expression of Bcl-xL in coronary endothelial cells under simulated ischaemia. Journal of Cellular and Molecular Medicine, 12(5A), 1584–1592.
Ryder, C., McColl, K., Zhong, F., & Distelhorst, C. W. (2012). Acidosis promotes Bcl-2 family-mediated evasion of apoptosis: involvement of acid-sensing G protein-coupled receptor Gpr65 signaling to Mek/Erk. Journal of Biological Chemistry, 287(33), 27863–27875.
Wojtkowiak, J. W., Rothberg, J. M., Kumar, V., Schramm, K. J., Haller, E., Proemsey, J. B., et al. (2012). Chronic autophagy is a cellular adaptation to tumor acidic pH microenvironments. Cancer Research, 72(16), 3938–3947.
Mizushima, N., & Klionsky, D. J. (2007). Protein turnover via autophagy: implications for metabolism. Annual Review of Nutrition, 27, 19–40.
Mani, S. A., Guo, W., Liao, M. J., Eaton, E. N., Ayyanan, A., Zhou, A. Y., et al. (2008). The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell, 133, 704–715.
Celià-Terrassa, T., Meca-Cortés, O., Mateo, F., de Paz, A. M., Rubio, N., Arnal-Estapé, A., et al. (2012). Epithelial-mesenchymal transition can suppress major attributes of human epithelial tumorinitiating cells. The Journal of Clinical Investigation, 122, 1849–1868.
Hjelmeland, A. B., Wu, Q., Heddleston, J. M., Choudhary, G. S., MacSwords, J., Lathia, J. D., et al. (2011). Acidic stress promotes a glioma stem cell phenotype. Cell Death and Differentiation, 18, 829–840.
Choi, S. Y., Collins, C. C., Gout, P. W., & Wang, Y. (2013). Cancer-generated lactic acid: a regulatory, immunosuppressive metabolite? The Journal of Pathology, 230(4), 350–355.
Lardner, A. (2001). The effects of extracellular pH on immune function. Journal of Leukocyte Biology, 69, 522–530.
Gottfried, E., Kunz-Schughart, L. A., Ebner, S., Mueller-Klieser, W., Hoves, S., Andreesen, R., et al. (2006). Tumor-derived lactic acid modulates dendritic cell activation and antigen expression. Blood, 107, 2013–2021.
Calcinotto, A., Filipazzi, P., Grioni, M., Iero, M., De Milito, A., Ricupito, A., et al. (2012). Modulation of microenvironment acidity reverses anergy in human and murine tumor-infiltrating T lymphocytes. Cancer Research, 72, 2746–2756.
Mendler, A. N., Hu, B., Prinz, P. U., Kreutz, M., Gottfried, E., & Noessner, E. (2012). Tumor lactic acidosis suppresses CTL function by inhibition of p38 and JNK/c-Jun activation. International Journal of Cancer, 131, 633–640.
Ohashi, T., Akazawa, T., Aoki, M., Kuze, B., Mizuta, K., Ito, Y., et al. (2013). Dichloroacetate improves immune dysfunction caused by tumor-secreted lactic acid and increases antitumor immunoreactivity. International Journal of Cancer, 133, 1107–1118.
Dhup, S., Dadhich, R. K., Porporato, P. E., & Sonveaux, P. (2012). Multiple biological activities of lactic acid in cancer: influences on tumor growth, angiogenesis and metastasis. Current Pharmaceutical Design, 18, 1319–1330.
Goetze, K., Walenta, S., Ksiazkiewicz, M., Kunz-Schughart, L. A., & Mueller-Klieser, W. (2011). Lactate enhances motility of tumor cells and inhibits monocyte migration and cytokine release. International Journal of Oncology, 9, 453–463.
Beckert, S., Farrahi, F., Aslam, R. S., Scheuenstuhl, H., Königsrainer, A., Hussain, M. Z., et al. (2006). Lactate stimulates endothelial cell migration. Wound Repair and Regeneration, 14, 321–324.
Végran, F., Boidot, R., Michiels, C., Sonveaux, P., & Feron, O. (2011). Lactate influx through the endothelial cell monocarboxylate transporter MCT1 supports an NF-κB/IL-8 pathway that drives tumor angiogenesis. Cancer Research, 71(7), 2550–2560.
Chen, J. L., Lucas, J. E., Schroeder, T., Mori, S., Wu, J., Nevins, J., et al. (2008). The genomic analysis of lactic acidosis and acidosis response in human cancers. PLoS Genetics, 4, e1000293.
Thews, O., Gassner, B., Kelleher, D. K., Schwerdt, G., & Gekle, M. (2006). Impact of extracellular acidity on the activity of P-glycoprotein and the cytotoxicity of chemotherapeutic drugs. Neoplasia, 8, 14352.
Raghunand, N., & Gillies, R. J. (2002). pH and drug resistance in tumors. Drug Resistance Updates, 3, 39–47.
Newell, K., Wood, P., Stratford, I., & Tannock, I. (1992). Effects of agents which inhibit the regulation of intracellular pH on murine solid tumours. British Journal of Cancer, 66, 311–317.
Ohtsubo, T., Igawa, H., Saito, T., Matsumoto, H., Park, H. J., Song, C. W., et al. (2001). Acidic environment modifies heat- or radiation-induced apoptosis in human maxillary cancer cells. International Journal of Radiation Oncology, Biology, and Physics, 49, 1391–8131.
Trowell, O. A. (1953). The effect of environmental factors on the radiosensitivity of lymph nodes cultured in vitro. British Journal of Radiology, 306, 302–309.
Haveman, J. (1980). The influence of pH on the survival after X-irradiation of cultured malignant cells. Effects of carbonylcyanide-3-chlorophenylhydrazone. International Journal of Radiation Biology, 37, 201–205.
Ohtsubo, T., Wang, X., Takahashi, A., Ohnishi, K., Saito, H., Song, C. W., et al. (1997). p53-dependent induction of WAF1 by a low-pH culture condition in human glioblastoma cells. Cancer Research, 57(18), 3910–3913.
Lee, H. S., Park, H. J., Lyons, J. C., Griffin, R. J., Auger, E. A., & Song, C. W. (1997). Radiation-induced apoptosis in different pH environments in vitro. International Journal of Radiation Oncology, Biology, and Physics, 38(5), 1079–1087.
Choi, E. K., Roberts, K. P., Griffin, R. J., Han, T., Park, H. J., Song, C. W., et al. (2004). Effect of pH on radiation-induced p53 expression. International Journal of Radiation Oncology, Biology, and Physics, 60, 1264–1271.
Park, H. J., Lee, S. H., Chung, H., Rhee, Y. H., Lim, B. U., Ha, S. W., et al. (2003). Influence of environmental pH on G2-phase arrest caused by ionizing radiation. Radiation Research, 159, 86–93.
Zhang, X., Lin, Y., & Gillies, R. J. (2010). Tumor pH and its measurement. Journal of Nuclear Medicine, 51(8), 1167–1170.
Delbeke, D., Coleman, R. E., Guiberteau, M. J., Brown, M. L., Royal, H. D., Siegel, B. A., et al. (2006). Procedure guideline for tumor imaging with 18F-FDG PET/CT 1.0. Journal of Nuclear Medicine, 47(5), 885–895.
Reshetnyak, Y. K., Andreev, O. A., Lehnert, U., & Engelman, D. M. (2006). Translocation of a molecules into cells by pH-dependent insertion of a transmembrane helix. Proceedings of the National Academy of Sciences of the United States of America, 103, 6460–6465.
Reshetnyak, Y. K., Segala, M., Andreev, O. A., & Engelman, D. M. (2007). A monomeric membrane peptide that lives in three worlds: in solution, attached to, and inserted across lipid bilayers. Biophysical Journal, 93, 2363–2372.
Andreev, O. A., Dupuy, A. D., Segala, M., Sandugu, S., Serra, D. A., Chichester, C. O., et al. (2007). Mechanism and uses of a membrane peptide that targets tumors and other acidic tissues in vivo. Proceedings of the National Academy of Sciences of the United States of America, 104, 7893–7898.
Vāvere, A. L., Biddlecombe, G. B., Spees, W. M., Garbow, J. R., Wijesinghe, D., Andreev, O. A., et al. (2009). A novel technology for the imaging of acidic prostate tumors by positron emission tomography. Cancer Research, 69(10), 4510–4516.
Aime, S., Botta, M., Crich, S. G., Giovenzana, G., Palmisano, G., & Sisti, M. (1999). A macromolecular Gd(III) complex as pH-responsive relaxometric probe for MRI applications. Chemical communications (Cambridge), 16, 1577–1578.
Zhang, S., Wu, K., & Sherry, A. D. (1999). A novel pH-sensitive MRI contrast agent. Angewandte Chemie International Edition in English, 38, 3192–3194.
Garcia-Martin, M. L., Martinez, G. V., Raghunand, N., Sherry, A. D., Zhang, S. R., & Gillies, R. J. (2006). High resolution pH(e) imaging of rat glioma using pH-dependent relaxivity. Magnetic Resonance in Medicine, 55, 309–315.
Robey, I. F., Baggett, B. K., Kirkpatrick, N. D., Roe, D. J., Dosescu, J., Sloane, B. F., et al. (2009). Bicarbonate increases tumor pH and inhibits spontaneous metastases. Cancer Research, 69, 2260–2268.
Silva, A. S., Yunes, J. A., Gillies, R. J., & Gatenby, R. A. (2009). The potential role of systemic buffers in reducing intratumoral extracellular pH and acid-mediated invasion. Cancer Research, 69, 2677–2684.
Ibrahim Hashim, A., Cornnell, H. H., Coelho Ribeiro Mde, L., Abrahams, D., Cunningham, J., Lloyd, M., et al. (2011). Reduction of metastasis using a non-volatile buffer. Clinical and Experimental Metastasis, 28, 841–849.
Fais, S., De Milito, A., You, H., & Qin, W. (2007). Targeting vacuolar H+-ATPases as a new strategy against cancer. Cancer Research, 67, 10627–10630.
De Milito, A., Canese, R., Marino, M. L., Borghi, M., Iero, M., Villa, A., et al. (2010). pH-dependent antitumor activity of proton pump inhibitors against human melanoma is mediated by inhibition of tumor acidity. International Journal of Cancer, 127, 207–219.
Yeo, M., Kim, D. K., Park, H. J., Cho, S. W., Cheong, J. Y., & Lee, K. J. (2008). Retraction: blockage of intracellular proton extrusion with proton pump inhibitor induces apoptosis in gastric cancer. Cancer Science, 99, 185.
Supino, R., Scovassi, A. I., Croce, A. C., Dal Bo, L., Favini, E., Corbelli, A., et al. (2009). Biological effects of a new vacuolar-H,-ATPase inhibitor in colon carcinoma cell lines. Annals of the New York Academy of Sciences, 1171, 606–616.
Lauritzen, G., Stock, C. M., Lemaire, J., Lund, S. F., Jensen, M. F., Damsgaard, B., et al. (2012). The Na+/H+ exchanger NHE1, but not the Na+, HCO3(−) cotransporter NBCn1, regulates motility of MCF7 breast cancer cells expressing constitutively active ErbB2. Cancer Letters, 317, 172–183.
He, B., Deng, C., Zhang, M., Zou, D., & Xu, M. (2007). Reduction of intracellular pH inhibits the expression of VEGF in K562 cells after targeted inhibition of the Na+/H+ exchanger. Leukemia Research, 2007(31), 507–514.
Provost, J. J., Rastedt, D., Canine, J., Ngyuen, T., Haak, A., Kutz, C., et al. (2012). Urokinase plasminogen activator receptor induced non-small cell lung cancer invasion and metastasis requires NHE1 transporter expression and transport activity. Cellular Oncology, 2012(35), 95–110.
Commisso, C., Davidson, S. M., Soydaner-Azeloglu, R. G., Parker, S. J., Kamphorst, J. J., Hackett, S., et al. (2013). Macropinocytosis of protein is an amino acid supply route in Ras-transformed cells. Nature, 497, 633–637.
Wong, P., Kleemann, H. W., & Tannock, I. F. (2002). Cytostatic potential of novel agents that inhibit the regulation of intracellular pH. British Journal of Cancer, 87(2), 238–245.
Harguindey, S., Arranz, J. L., Polo Orozco, J. D., Rauch, C., Fais, S., Cardone, R. A., et al. (2013). Cariporide and other new and powerful NHE1 inhibitors as potentially selective anticancer drugs—an integral molecular/biochemical/metabolic/clinical approach after one hundred years of cancer research. Journal of Translational Medicine, 11(1), 282.
Gao, W., Chang, G., Wang, J., Jin, W., Wang, L., Lin, Y., et al. (2011). Inhibition of K562 leukemia angiogenesis and growth by selective Na+/H+ exchanger inhibitor cariporide through down-regulation of pro-angiogenesis factor VEGF. Leukemia Research, 11, 1506–1511.
Sennoune, S. R., Luo, D., & Martínez-Zaguilán, R. (2004). Plasmalemmal vacuolar-type H+-ATPase in cancer biology. Cell Biochemistry and Biophysics, 40(2), 185–206.
Luciani, F., Spada, M., De Milito, A., Molinari, A., Rivoltini, L., Montinaro, A., et al. (2004). Effect of proton pump inhibitor pretreatment on resistance of solid tumors to cytotoxic drugs. Journal of the National Cancer Institute, 96, 1702–1713.
Shen, Y., Wu, Y., Chen, M., Shen, W., Huang, S., Zhang, L., et al. (2012). Effects of pantoprazole as a HIF-1α inhibitor on human gastric adenocarcinoma sgc-7901 cells. Neoplasma, 59, 142–149.
Wahl, M. L., Owen, J. A., Burd, R., Herlands, R. A., Nogami, S. S., Rodeck, U., et al. (2002). Regulation of intracellular pH in human melanoma: potential therapeutic implications. Molecular Cancer Therapeutics, 1(8), 617–628.
Supuran, C. T. (2008). Carbonic anhydrases: novel therapeutic applications for inhibitors and activators. Nature Reviews Drug Discovery, 7, 168–181.
Acknowledgments
We acknowledge the “Istituto Toscano Tumori” and Ente Cassa di Risparmio di Firenze for their financial support.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Peppicelli, S., Bianchini, F. & Calorini, L. Extracellular acidity, a “reappreciated” trait of tumor environment driving malignancy: perspectives in diagnosis and therapy. Cancer Metastasis Rev 33, 823–832 (2014). https://doi.org/10.1007/s10555-014-9506-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10555-014-9506-4