Abstract
Purpose
To investigate the association between external beam radiotherapy (EBRT) for prostate cancer and mesothelioma using data from the US Surveillance, Epidemiology, and End Results (SEER) cancer registries.
Methods
We analyzed data from the SEER database (1973–2009). We compared EBRT versus no radiotherapy. Incidence rate ratios (IRR) and 95 % confidence intervals (95 % CI) of mesothelioma among prostate cancer patients were estimated with multilevel Poisson models adjusted by race, age, and calendar year. Confounding by asbestos was investigated using relative risk of mesothelioma in each case’s county of residence as a proxy for asbestos exposure.
Results
Four hundred and seventy-one mesothelioma cases (93.6 % pleural) occurred in 3,985,991 person-years. The IRR of mesothelioma was increased for subjects exposed to EBRT (1.28; 95 % CI 1.05, 1.55) compared to non-irradiated patients, and a population attributable fraction of 0.49 % (95 % CI 0.11, 0.81) was estimated. The IRR increased with latency period: 0–4 years, IRR 1.08 (95 % CI 0.81, 1.44); 5–9 years, IRR 1.31 (95 % CI 0.93, 1.85); ≥10 years, IRR 1.59 (95 % CI 1.05, 2.42). Despite the fairly strong evidence of association with EBRT, the population attributable rate of mesothelioma was modest—3.3 cases per 100,000 person-years. The cumulative incidence of mesothelioma attributable to EBRT was 4.0/100,000 over 5 years, 24.5/100,000 over 10 years, and 65.0/100,000 over 15 years.
Conclusions
Our study provides evidence that EBRT for prostate cancer is a small but detectable risk factor for mesothelioma. Patients should be advised of risk of radiation-induced second malignancies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Malignant mesothelioma is a cancer originating from the lining cells of the pleural and peritoneal cavities, as well as the pericardium and the tunica vaginalis. Although mesothelioma is a rare cancer, its incidence has increased steeply from the 1970s through the mid-1990s [1].
Mesothelioma is primarily a disease of adults and usually presents in the fifth to seventh decades, and 70–80 % of cases occur in men [2]. Occupational, para-occupational, and non-occupational environmental exposures to asbestos are the major risk factor for mesothelioma [2]. In Western countries, the proportion of mesothelioma cases with an identified asbestos exposure have been found to be around 80–90 % in men and 50–60 % in women [3, 4]. The fraction of mesothelioma attributable to occupational or para-occupational exposure in Britain was estimated as 97.0 % (95 % CI 96.0, 98.0 %) for males and 82.5 % (95 % CI 75.0, 90.0 %) for females [5]. Even though asbestos is the main risk factors for mesothelioma and some authors have speculated that “virtually all mesotheliomas are due to asbestos exposure, and most exposure is related to work” [6], a background lifetime probability (i.e., the probability that would be expected in the absence of exposure to asbestos) of about 3 per 10,000 has been estimated [1, 7]. Additional exposures that have been hypothesized, with varying degrees of evidence, to be causal factors of mesothelioma include non-asbestiform mineral fibers (erionite; fluoro-edenite); carbon nanotubes; viruses (MC29 avian leukosis virus; SV40); metals; chronic serosal inflammation; and ionizing radiation [8].
Ionizing radiation sources linked (again with varying amounts of evidence) to mesothelioma are exposure to the diagnostic X-ray contrast medium “Thorotrast,” working in nuclear power plants, and external beam radiotherapy (EBRT) [9]. The association between radiotherapy and mesothelioma was initially hypothesized after numerous case reports of mesotheliomas occurring after irradiation of nearby organs [9]. Goodman and colleagues reviewed the evidence linking EBRT to mesothelioma; all available studies were retrospective cohort analyses of cancer registry data. Most studies used data from the US National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) Program database and studied primary neoplasms characterized by long survival and treatment with EBRT (cancers of the breast and testis as well as Hodgkin’s and non-Hodgkin’s lymphomas). Positive associations between EBRT and mesothelioma were reported in most of the studies reviewed by Goodman, but the findings were limited by a high degree of variability due to the small number of mesothelioma cases analyzed (maximum 40 cases, often <15) [9].
Prostate cancer is the most common cancer among males in the USA with more than 240,000 diagnoses expected in 2012 [10]. EBRT has long been the standard option for the treatment for locally advanced prostate cancer [11]. As many men are exposed every year to EBRT for the treatment for prostate cancer, considerable attention has been paid to the risk of radiation-induced second malignancies; with most of the emphasis understandably placed on risks of second cancers in sites proximal to the irradiated area (e.g., colorectal and bladder cancer). There have, however, been three studies linking EBRT for prostate cancer to increased risk of lung cancer [12–14]. To our knowledge, no study has been conducted on the association between EBRT for the treatment for prostate cancer and the risk of mesothelioma.
The aim of this study was to investigate the possible association between EBRT for primary prostate cancer and the risk of malignant mesothelioma using data from the SEER registries.
Methods
Population and follow-up
We defined the cohort as patients who were diagnosed with a first primary invasive prostate cancer reported to one of the SEER registries. The SEER 9 Registries database (including Atlanta, Connecticut, Detroit, Hawaii, Iowa, New Mexico, San Francisco-Oakland, Seattle-Puget Sound, and Utah) was consulted for the period between 1 January 1973 and 31 December 1991, while the SEER 13 Registries database (which further includes San Jose-Monterey, Los Angeles, Alaska Natives, and Rural Georgia) was used for the period between 1 January 1992 and 31 December 2009. Individual records were obtained from MP-SIR (Multiple Primary-Standardized Incidence Ratios) session of SEER*Stat software. The Alaska Natives registry was excluded from our analysis as no cancer record is present in the MP-SIR session.
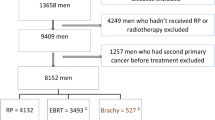
One case diagnosed after death on the grounds of death certificate/autopsy was excluded from the study population. The follow-up time for each individual started at the date of the diagnosis of prostate cancer and ended at the date of the diagnosis of mesothelioma, at last known vital status, death, or the end of the study (31 December 2009). To reduce the misclassification of exposure to EBRT, patients with another record in the SEER database before the one bearing the diagnosis of prostate cancer were excluded from the study. We also excluded patients with missing information on radiotherapy or on county of residence (Fig. 1 summarizes the study population).
Exposure and covariates
Available information on the first course of treatment allowed us to classify patients according to whether or not they had received radiotherapy as a part of their initial treatment for prostate cancer. Hence, using the information from SEER registries, the patients were classified into three groups as follows:
-
1.
no radiotherapy;
-
2.
EBRT alone or in combination with brachytherapy;
-
3.
radioactive implants, radioisotopes, other forms of radiation when the method or source was not specified.
The incidence rate ratios (IRR) of mesothelioma for patients treated with radiotherapy (groups 2 and 3 above) were estimated with reference to patients who did not undergo radiotherapy. We also performed a sensitivity analysis in which the IRRs were estimated with respect to the subset of non-irradiated patients who received surgical treatment for their cancer.
Covariates to be considered in multivariate analysis were selected a priori and included age (completed years), calendar year, and race (white, black, other). As noted, asbestos exposure is known to be the major cause of mesothelioma, and as such might potentially represent a confounder of the association between EBRT and the tumor. Unfortunately, no individual work history or other exposure information is available in SEER, and so we used a geographic analysis to investigate possible confounding by asbestos exposure. Many important industrial uses of asbestos tended in the past to be clustered (e.g., shipyards, asbestos textile plants), and as a result, mesotheliomas have been observed to cluster as well [15]. Moreover, the authors of a recent Italian study of municipal clusters of mesothelioma concluded that “pleural mesothelioma mortality at population level is a suitable indicator of previous asbestos exposure” [16]. We conducted an analysis of mesothelioma rates by county in the USA using the SEER 13 Registries data (1992–2009), and then linked each case’s county of residence to that county’s relative risk of mesothelioma as a proxy for county-level asbestos exposure. A three-level variable (low, medium, and high relative risk of mesothelioma among men) was created using tertiles of the distribution of the county mesothelioma relative risks. Each subject was classified according to the county of residence at the time of the diagnosis of prostate cancer.
In previous studies on radiation-induced second malignancies, tumor grade and stage have sometimes been included among the covariates (e.g., Moon et al. [13]). However, prostate cancer grade and stage are unlikely to be associated with the risk of mesothelioma; furthermore, the information on stage is often unavailable for SEER records [17]. Hence, we excluded tumor grade and stage from the covariates; nevertheless, we performed a sensitivity analysis restricted to subjects with full information to explore the role of grade and stage in the causal pathway between EBRT and mesothelioma.
Latency (time since first exposure to EBRT) was considered as a possible effect modifier of the relationship between EBRT and mesothelioma: based on current knowledge of carcinogenesis, it is likely that a minimum period of 5 years is necessary for the induction of solid cancers after exposure to radiation [18]. Latency was calculated with reference to the date of the diagnosis of prostate cancer (presumed to be shortly before EBRT exposure). Our ability to investigate particularly long latencies was limited by our data: only 30 cases of mesothelioma in 120,731 person-years were observed 15 or more years after the primary diagnosis of prostate cancer. Consequently, latency was grouped into three categories: 0–4 years; 5–9 years; and 10 or more years.
Outcome measures and case definitions
The main outcome measure was the incidence rate ratio (IRR) of mesothelioma in patients exposed to EBRT compared to patients unexposed to radiotherapy after primary prostate cancer. We also estimated the incidence rate difference (IRD, sometimes called attributable rate) of mesothelioma to provide perspective on the absolute magnitude of the risk from EBRT. Due to the small number of extrapleural mesotheliomas in the study population, we focused our main analysis on all cases of mesothelioma, irrespective of cancer site.
We estimated the population attributable fraction (PAF), that is, the proportion by which the incidence rate of the outcome in the entire population would be reduced if the exposure was eliminated, [19] to evaluate the role of EBRT in the global mesothelioma epidemic.
Statistical analysis
The IRRs of mesothelioma and 95 % confidence intervals (95 % CIs) were estimated using Poisson regression models. Temporal variables (i.e., age, calendar year, and latency) were analyzed as time-varying variables. We performed analysis stratified by latency categories. Based on preliminary analysis, two parameters were introduced in the multivariate models for age (completed years): age and squared age. Calendar year was introduced in the models with one degree of freedom.
When adjusting for county of residence RR of mesothelioma, we used multilevel random intercept Poisson regression models accounting for the within-county variance component. The models assumed a γ distribution of the county-level random intercept, accounting also for within-county dependence [20].
The relative risks of mesothelioma were calculated for the counties covered by the 13 SEER registries using the Besag–York–Mollie (BYM) model [21]. Briefly, the BYM model allows for both heterogeneous and spatially structured random effects; additional details on the model are presented in Web Appendix 1 along with the results of the analysis summarized in maps of the standardized incidence ratios and RRs (Web Figures 1 and 2).
We estimated the adjusted incidence rate difference (IRD) of mesothelioma (and 95 % CIs) for different treatments (EBRT, other radiation, and none) and for tertiles of county mesothelioma RR by applying the model proposed by Xu and colleagues [22]. The method consists of an ordinary least-squares regression of transformed variables, together with a robust variance estimator for inference; the calculated coefficients are an unbiased estimate of the IRDs at the group level [22]. As the method by Xu and colleagues was presented for analyzing categorical variables, we aggregated ages and calendar years in categories (as presented in Table 1) when estimating IRDs.
The population attributable fraction (PAF) was calculated for EBRT as\( {\text{PAF}} = P_{\text{c}} *\left( {{\text{RR}} - 1} \right)/{\text{RR}}\)where P c is the proportion of exposed among cases (here, the proportion of mesotheliomas following prostate cancer who received EBRT). The confidence interval for the PAF was estimated via Monte Carlo simulation assuming a binomial distribution for the P c and lognormal for the RR.
Figures on cancer and individual records were obtained using SEER*Stat software version 7.0.9. We used WinBugs 1.4.3 to fit the BYM model and Stata 11.2 SE (Stata Corporation, Texas, TX, USA) software package for the main analysis.
Equations developed by Kry and colleagues were used to estimate the equivalent absorbed radiation doses at two pleural sites following two different standard EBRT techniques for prostate cancer [23]. Information needed to apply the equations is as follows: (1) distance from the treatment field; (2) depth of the tissue; and (3) treatment strategy. We assumed a distance ranging between 27.5(lung edge) and 35 cm (lung center) and a depth of 3 cm [24]. We estimated the equivalent adsorbed radiation dose for exposure to three-dimensional conformal radiation therapy (18 megavolt, 78.0 Gray) or to intensity-modulated radiation therapy (6 megavolt, 75.6 Gray; 18 megavolt, 75.6 Gray).
Results
There were 583,974 cases of primary prostate cancer reported to one of the consulted SEER registries during the study period. After the exclusion of patients without follow-up (4,615), with another record before the one bearing the diagnosis of prostate cancer (107), or with missing information on radiotherapy (8,200) or county (169), we identified a cohort of 570,883 subjects diagnosed with primary prostate cancer (Fig. 1). The final study population included 3,985,991 person-years and 471 cases of mesothelioma (441 localized to the pleura, 21 to the peritoneum, and 9 to other or unspecified sites).
Diagnostic techniques used for the identification of mesothelioma were as follows: histology (n = 391); exfoliative cytology (57); direct visualization (3); radiography (11); and clinical diagnosis (7). Quality of diagnosis was unknown for 2 cases.
A summary of the cohort (Table 1) reveals important differences in use of radiotherapy for prostate cancer among races, age groups, and over time. The use of other radiotherapy techniques (including radioactive implants) increased within the study period and was more frequent among white patients; 5.2 % of white patients received other radiotherapy techniques, compared to 3.8 % of black patients and 4.0 % of other non-white, non-black patients. Patients aged between 70 and 85 were more likely to be exposed to EBRT than to other forms of radiation.
An increased risk of mesothelioma was observed for patients exposed to EBRT compared to those who received no radiotherapy (adjusted IRR 1.28, 95 % CI 1.05, 1.55), while no increase in risk was seen from other radiotherapies (Table 2). Compared to white men, blacks, and other races had a substantially lower risk of mesothelioma following prostate cancer, independent of the risk from radiotherapy. Men with prostate cancer and residing in a county with a mesothelioma incidence rate in the highest tertile had about a threefold increased risk of mesothelioma (IRR 2.98; 95 % CI 1.76, 5.03), again independent of the choice of radiotherapy. A dose–response relationship across the tertiles of county mesothelioma risk was apparent (p for trend <0.001). Although the county’s mesothelioma RR was able to predict the individual resident’s risk of mesothelioma, it was not a confounder of the relationship between EBRT and mesothelioma: the IRR for EBRT in the multivariate model in Table 2 was unchanged to 3 decimal places after removing the county mesothelioma risk (data not shown). As expected, age was a strong determinant of mesothelioma incidence (Web Figure 3). Also, the risk of mesothelioma increased with calendar year (adjusted IRR 1.01; 95 % CI 1.00, 1.03).
As shown in Table 3, the adjusted IRD of mesothelioma for patients exposed to EBRT was 3.31 per 100,000 person-years (95 % CIs 0.72, 5.90). This figure can be compared to the incidence rate increase of 9.69 per 100,000 person-years (95 % CI 6.91, 12.47) for subjects resident in counties with an RR of mesothelioma in the top third, compared to those in the lowest third.
Complete information on tumor grade and stage were available for only 321,041 prostate cancers recorded between 1988 and 2009. The IRR of mesothelioma for subjects exposed to EBRT increased only by 2 % after adjusting by tumor stage and grade and neither of these two classifications showed any evidence of association with mesothelioma risk (see Web Table 1). The IRR of mesothelioma for EBRT estimated in the sensitivity analysis conducted using non-irradiated patients who received surgery as the comparison group was identical to that obtained when using all the non-irradiated patients as the reference category (Web Table 2).
The strength of the association between EBRT and mesothelioma increased with increasing latency period (Fig. 2). For latency from zero to 4 years, the IRR was 1.08 (95 % CI 0.81, 1.44) and the IRD was 0.9 per 100,000 pyrs (95 % CI −2.4, 4.2); for latency from 5 to 9 years, the IRR was 1.31 (95 % CI 0.93, 1.85) and the IRD was 4.0 per 100,000 pyrs (95 % CI −0.8, 8.9); and for latency of ten or more years, the IRR was 1.59 (95 % CI 1.05, 2.42) and the IRD was 8.1 per 100,000 pyrs (95 % CI 0.6, 15.7). Based on the observed IRDs, we estimated a cumulative incidence of mesothelioma attributable to EBRT of 4.0/100,000 over 5 years, of 24.5/100,000 over 10 years, and of 65.0/100,000 over 15 years.
Incidence rate ratios (95 % confidence intervals) of mesothelioma for subjects exposed to external beam radiotherapy compared to subjects not exposed to radiotherapy after prostate cancer. Analysis stratified by latency period. SEER 9 Registries (1973–1991), SEER 13 Registries (1992–2009). Incidence rate ratios for all mesothelioma sites were estimated with multilevel Poisson regression model adjusted by race, age, age2, year, and county’s mesothelioma relative risk
Only 21 of the 471 mesothelioma cases considered in the present analysis were localized at the peritoneum; hence, we did not perform multivariate analysis separately for peritoneal mesothelioma. Univariate analyses like those shown in Table 2 but limited to peritoneal mesothelioma showed very similar patterns, albeit with wide confidence intervals. The incidence rate ratio for those exposed to EBRT was (IRR 1.75, 95 % CI 0.74, 4.15). Risk appeared to rise with increasing latency as well, although again the confidence intervals were wide (Fig. 2). The IRR of peritoneal mesothelioma was 1.16 (95 % CI 0.35, 3.87) for latency periods shorter than 5 years, 2.21 (95 % CI 0.31, 15.71) for latency periods between 5 and 9 years, and 3.87 (95 % CI 0.65, 23.14) for latency periods of 10 years or more.
We estimated the population attributable fraction (PAF) for EBRT contributing to mesothelioma using data for the 7,450 cases of mesothelioma among males recorded in the SEER 9 Registries or in the SEER 13 Registries (excluding the Alaska Natives registry). For the same time period, we identified 168 mesothelioma cases that had been exposed to EBRT for treating prostate cancer, yielding an exposure prevalence among cases of 2.3 % (95 % CIs 1.9, 2.6 %). Assuming a causal association, the PAF can be calculated from this exposure prevalence and from the IRR of mesothelioma for exposure to EBRT after prostate cancer (IRR 1.28, 95 % CIs 1.05, 1.55) in Table 2. We estimated that 0.49 % (95 % CI 0.11, 0.81 %) of mesothelioma cases resulted from EBRT after primary prostate cancer.
Using the equations of Kry and colleagues, the estimated equivalent absorbed radiation doses to the pleura from EBRT ranged from about 7–25 milliSeverts (Web Table 3).
Discussion
We found an increased risk of mesothelioma in subjects that were previously irradiated to treat prostate cancer. The risk appeared to increase with latency. Based on the closer proximity to the site of irradiation, one might expect the risk to be higher for peritoneal mesotheliomas. Within the limits of the small number of such cases, this hypothesis appears to be supported—the point estimate for peritoneal mesothelioma risk and EBRT exposure was higher albeit with wide confidence intervals, and the IRRs stratified according to latency presented the same pattern found when analyzing all mesotheliomas combined.
Previous evidence and biological plausibility
Our findings support an association between exposure to EBRT and risk of mesothelioma. This observation is predominantly based on pleural mesotheliomas (accounting for more than 93 % of the cases) which occur distant from the irradiation field for prostate cancer.
Radiation-induced malignancies are usually expected to occur within the irradiated field (e.g., Baxter et al. [25]). However, even organs far from the irradiated field can still be significantly exposed due to scattered radiation, as well as leakage from the radiation source [23, 26, 27]. Three-dimensional conformal radiation therapy of the prostate (a frequent treatment during the 1990s [28]) can expose the pleura to an equivalent absorbed radiation dose up to 25 mSv (Web Table 3); this value is far from being insignificant if we consider that the effective dose for a standard chest radiograph ranges between 0.05 and 0.24 mSv [29]. When interpreting this value, we should consider that 0.6–1.8 % of the cumulative risk of cancer to age 75 years could be attributable to diagnostic X-rays [30].
Findings from registry-based studies on second cancers provided inconsistent evidence. On the one hand, previous studies highlighted a possible association between EBRT for prostate cancer and risk of lung cancer [12–14]. On the other hand, a study on second neoplasms after invasive breast cancer did not identify any increase in risk for medium (0.5–1.0 Gy) or low (below 0.5 Gy) doses of radiation [31]. However, it is interesting to note that among sites receiving high radiation doses, pleural cancers presented the highest point estimate, although based on only two cases [17].
Our study period was limited (1973–2009), and we studied latencies shorter than those usually reported for asbestos-related mesothelioma [32]. Nevertheless, previous studies of the latency period of radiation-induced solid tumors suggest an average latency period of 5–15 years, in line with our analysis [14, 18]. Furthermore, several case reports on EBRT and mesothelioma have described cases occurring after latency periods in the range of 5–41 years [9].
Epidemiologic evidence that might support an association between exposure to ionizing radiation and mesothelioma is inconsistent. On the one hand, many studies on Thorotrast or EBRT and risk of mesothelioma do report increased risk of mesothelioma among subjects exposed to radiation [9]. On the other hand, studies among occupational cohorts working in the nuclear industry have not observed increased risk of mesothelioma, at least not that can be confidently attributed to radiation exposure rather than to confounding by asbestos [9, 33].
Study strength and limitations
Our study was based on a large number of mesothelioma cases, in contrast with previous studies on EBRT and mesothelioma [9]. Hence, we were able to detect a small increase in risk (about 30 %) and to conduct an analysis stratified according to latency.
The main limitation of our study is the potential for unmeasured confounding as information on personal characteristics and individual exposures was lacking. Confounding due to exposure to asbestos is always a concern when studying mesothelioma. In the present analysis, we were unable to adjust our estimates according to the personal history of exposure. Instead, we used the RR of mesothelioma among males in the county of residence as a proxy measure of exposure to asbestos. Although certainly affected by a high degree of misclassification, this measure of exposure to asbestos was able to capture at least part of the individual risk of mesothelioma, as shown by the well-shaped dose–response relationship. It is important to note that the estimate of interest, that is, the IRR for exposure to EBRT, did not change after the introduction in the multivariate models of the variable for the county’s RR of mesothelioma. This finding suggests that the association between EBRT and mesothelioma was not highly confounded by asbestos in our study population.
It is possible that receiving radiotherapy rather than surgery might in some way be associated with determinants of mesothelioma, although aside from asbestos, there are no established personal risk factors for mesothelioma that could represent a contraindication for surgery. It is possible that there was a higher prevalence of chronic cardiac and pulmonary diseases among people occupationally exposed to asbestos because the asbestos exposure tends to occur more often in lower socioeconomic classes where poorer overall health might increase the aforementioned chronic conditions [34–36]. Treatment decision making in prostate cancer is influenced by the presence of comorbidities; patients affected by chronic diseases have a higher probability of receiving radiotherapy instead of surgery [37]. Hence, our findings could be at least partially explained by a higher tendency for former asbestos workers to receive EBRT (although we have no direct evidence to suggest this). To explore this possible source of selection bias, we performed a supplemental analysis in which only patients who had received neither surgery nor radiotherapy were used as the comparison group (Web Table 2). This change did not alter the point estimates of the risk associated with EBRT, although the smaller comparison group necessarily resulted in larger standard errors. Reports on the distribution of socioeconomic factors (i.e., level of education and income) highlighted that patients from lower socioeconomic status were less likely to receive any treatments, whereas the percentage of patients receiving radiation was usually independent from socioeconomic status or even showed a positive association [38–42]. Therefore, while the comparison between surgery and radiotherapy could be affected by a bias away from the null hypothesis (i.e., showing a risk greater than the real one), the comparison between patients who received surgery and patients who did not receive therapies should be biased toward the null hypothesis (i.e., showing a risk smaller than the real one). It is also interesting that the IRR for patients who received only surgery compared to untreated patients was 1.00 (Web Table 2); this observation suggests that the distribution of previous occupational exposure to asbestos according to treatment status is not likely to be importantly unbalanced.
We also repeated this analysis after excluding stage I cancers, a subpopulation in which active surveillance has been proposed in the absence of comorbidities [43]. The risk for patients treated with EBRT compared to patients who did not receive any therapy was still close to that estimated in the main analysis (IRR = 1.28, data not shown). Taken together these findings suggest that selection bias introduced by comorbidities associated with asbestos exposure is not a major concern in our study. Another element supporting the absence of confounding by asbestos exposure is the increase in risk with latency (Fig. 2). Our estimates were adjusted by age and squared age, which were introduced in the models as time-varying covariates; under these conditions, residual confounding by age is unlikely. Moreover, almost no increased risk was observed in the first 5 years after the irradiation. Hence, the latency period from the diagnosis of prostate cancer is unlikely to reflect an age-dependent latency from the first occupational exposure to asbestos. To produce the observed pattern of estimates, confounding by asbestos should act differently in each latency period.
We also performed a target-adjustment sensitivity analysis to explore the difference in prevalence of occupational exposure to asbestos by EBRT status that would have been necessary to explain the observed associations (see Web Appendix 1). In order to completely explain the IRR observed for EBRT, the prevalence of occupational exposure to asbestos would have to have been 30 % higher in subjects exposed to EBRT compared to unexposed subjects (see Web Table 4). And, when applying a more plausible assumption of a latency of 10 or more years, the EBRT group would have to have had 63 % higher asbestos exposure than the comparison group in order to fully explain the observed association between EBRT and mesothelioma. Such large differences, with no evident explanation, are implausible.
Registry-based studies of cancer might be affected by detection bias (also called surveillance bias). Detection bias occurs when there are systematic differences between the study groups in the assessment of the outcome [19]. This kind of bias is often a serious threat when studying cancers that can be clinically silent for a long period (e.g., prostate cancer or breast cancer). Indeed, clinical follow-up of the primary cancer may elicit the identification of secondary neoplasms that otherwise would have gone undetected. The risk of mesothelioma for subjects treated with EBRT observed in our study increased with latency period; the higher risk was found for latency of 10 years or more. A substantial difference in health monitoring among the studied groups is unlikely to have occurred so far from the primary treatment for prostate cancer. Also, an analysis restricted to patients aged up to 85 years, a subpopulation in which under-ascertainment is less likely in SEER data [44], produced estimates similar to those obtained when studying the entire population (IRR of mesothelioma 1.24, 95 % CI 1.01, 1.53—data not shown). On balance, we believe that detection bias was not likely to have been a major limitation in our study.
As in some previous analyses of SEER data, we did not adjust by tumor stage and grade. However, a sensitivity analysis confirmed our a priori hypothesis that grade and stage of prostate cancer did not appear to be confounders of the association between EBRT and mesothelioma.
We do not believe that the quality of diagnosis is a serious limitation. Most of the mesothelioma cases were diagnosed with histology or exfoliative cytology which are effective in the differential diagnosis with peripheral lung cancer. On the other hand, no information was provided in SEER on results of an immunohistochemical panel, which has been recommended to distinguish benign from malignant mesothelial proliferations [45]. We cannot therefore exclude the possibility that the incidence of mesothelioma may have been slightly over-diagnosed; however, this misdiagnosis is unlikely to have been differential with respect to the exposure of interest (beam radiotherapy).
Exposure to EBRT was studied as a dichotomous variable as no detailed information on radiation doses or treatment type was available; therefore, we were not able to study a dose–response relationship. Also, it is likely that there was a certain degree of exposure misclassification because some subjects classified as unexposed at the baseline may have undergone EBRT later in the study period. This source of non-differential misclassification of exposure is likely to have biased estimate toward the null hypothesis.
When modeling on the absolute scale, we found that the increase in risk was modest (3.31 per 100,000 person-years); as a point of comparison, we note that it is smaller than that determined by living in a county at high risk of mesothelioma. This perspective and the very small population attributable fraction (0.49 %) suggest that the contribution of EBRT to the worldwide mesothelioma epidemic has been negligible.
Conclusions
Our study provides evidence that EBRT for prostate cancer is a risk factor for mesothelioma. However, we found a small absolute increase in risk and a small population attributable fraction; hence, we believe that EBRT is unlikely to have played any significant role in the global mesothelioma epidemic. Our findings corroborate the hypothesis that EBRT could be a risk factor not only for cancer sites proximal to the primary treated tumor, but also for radiogenic cancers throughout the body [46].
References
Price B, Ware A (2004) Mesothelioma trends in the United States: an update based on surveillance, epidemiology, and end results program data for 1973 through 2003. Am J Epidemiol 159:107–112. doi:10.1093/aje/kwh025
Moore AJ, Parker RJ, Wiggins J (2008) Malignant mesothelioma. Orphanet J Rare Dis 3:34. doi:10.1186/1750-1172-3-34
Goldberg M, Imbernon E, Rolland P, Gilg Soit Ilg A, Savès M, de Quillacq A, Frenay C, Chamming’s S, Arveux P, Boutin C, Launoy G, Pairon JC, Astoul P, Galateau-Sallé F, Brochard P (2006) The French national mesothelioma surveillance program. Occup Environ Med 63:390–395. doi:10.1136/oem.2005.023200
Rake C, Gilham C, Hatch J, Darnton A, Hodgson J, Peto J (2009) Occupational, domestic and environmental mesothelioma risks in the British population: a case-control study. Br J Cancer 100:1175–1183. doi:10.1038/sj.bjc.6604879
Rushton L, Bagga S, Bevan R, Brown TP, Cherrie JW, Holmes P, Fortunato L, Slack R, Van Tongeren M, Young C, Hutchings SJ (2010) Occupation and cancer in Britain. Br J Cancer 102:1428–1437. doi:10.1038/sj.bjc.6605637
Driscoll T, Nelson DI, Steenland K, Leigh J, Concha-Barrientos M, Fingerhut M, Prüss-Ustün A (2005) The global burden of disease due to occupational carcinogens. Am J Ind Med 48:419–431. doi:10.1002/ajim.20209
Moolgavkar SH, Meza R, Turim J (2009) Pleural and peritoneal mesotheliomas in SEER: age effects and temporal trends, 1973–2005. Cancer Causes Control 20:935–944. doi:10.1007/s10552-009-9328-9
Jasani B, Gibbs A (2012) Mesothelioma not associated with asbestos exposure. Arch Pathol Lab Med 136:262–267. doi:10.5858/arpa.2011-0039-RA
Goodman JE, Nascarella MA, Valberg PA (2009) Ionizing radiation: a risk factor for mesothelioma. Cancer Causes Control 20:1237–1254. doi:10.1007/s10552-009-9357-4
Siegel R, Naishadham D, Jemal A (2012) Cancer statistics, 2012. CA Cancer J Clin 62:10–29. doi:10.3322/caac.20138
Damber JE, Aus G (2008) Prostate cancer. Lancet 2008(371):1710–1721. doi:10.1016/S0140-6736(08)60729-1
Bhojani N, Capitanio U, Suardi N, Jeldres C, Isbarn H, Shariat SF, Graefen M, Arjane P, Duclos A, Lattouf JB, Saad F, Valiquette L, Montorsi F, Perrotte P, Karakiewicz PI (2010) The rate of secondary malignancies after radical prostatectomy versus external beam radiation therapy for localized prostate cancer: a population-based study on 17,845 patients. Int J Radiat Oncol Biol Phys 76:342–348. doi:10.1016/j.ijrobp.2009.02.011
Moon K, Stukenborg GJ, Keim J, Theodorescu D (2006) Cancer incidence after localized therapy for prostate cancer. Cancer 107:991–998. doi:10.1002/cncr.22083
Brenner DJ, Curtis RE, Hall EJ, Ron E (2000) Second malignancies in prostate carcinoma patients after radiotherapy compared with surgery. Cancer 88:398–406. doi:10.1002/(SICI)1097-0142
Enterline PE, Henderson VL (1987) Geographic patterns for pleural mesothelioma deaths in the United States, 1968–81. J Natl Cancer Inst 79:31–37. doi:10.1093/jnci/79.1.31
Fazzo L, De Santis M, Minelli G, Bruno C, Zona A, Marinaccio A, Conti S, Comba P (2012) Pleural mesothelioma mortality and asbestos exposure mapping in Italy. Am J Ind Med 55:11–24. doi:10.1002/ajim.21015
Berrington de Gonzalez A, Curtis RE, Kry SF, Gilbert E, Lamart S, Berg CD, Stovall M, Ron E (2011) Proportion of second cancers attributable to radiotherapy treatment in adults: a cohort study in the US SEER cancer registries. Lancet Oncol 12:353–360. doi:10.1016/S1470-2045(11)70061-4
Preston DL, Ron E, Tokuoka S, Funamoto S, Nishi N, Soda M, Mabuchi K, Kodama K (2007) Solid cancer incidence in atomic bomb survivors: 1958–1998. Radiat Res 168:1–64. doi:10.1667/RR0763.1
Porta M (2008) A dictionary of epidemiology. Oxford University Press, New York
Rabe-Hesketh S, Skrondal A (2008) Multilevel and longitudinal modeling using stata. Stata Press, College Station
Besag J, York J, Mollie A (1991) Bayesian image restoration, with two applications in spatial statistics (with discussion). Ann Inst Stat Math 43:1–59. doi:10.1007
Xu Y, Cheung YB, Lam KF, Tan SH, Milligan P (2010) A simple approach to the estimation of incidence rate difference. Am J Epidemiol 172:334–343. doi:10.1093/aje/kwq099
Kry SF, Salehpour M, Followill DS, Stovall M, Kuban DA, White RA, Rosen II (2005) Out-of-field photon and neutron dose equivalents from step-and-shoot intensity-modulated radiation therapy. Int J Radiat Oncol Biol Phys 62:1204–1216. doi:10.1016/j.ijrobp.2004.12.091
Wax DB, Leibowitz AB (2007) Radiologic assessment of potential sites for needle decompression of a tension pneumothorax. Anesth Analg 105:1385–1388. doi:10.1213/01.ane.0000282827.86345.ff
Baxter NN, Tepper JE, Durham SB, Rothenberger DA, Virnig BA (2005) Increased risk of rectal cancer after prostate radiation: a population-based study. Gastroenterology 128:819–824. doi:10.1053/j.gastro.2004.12.038
Francois P, Beurtheret C, Dutreix A (1988) Calculation of the dose delivered to organs outside the radiation beams. Med Phys 15:879–883. doi:10.1118/1.596170
Hall EJ, Wuu CS (2003) Radiation-induced second cancers: the impact of 3D-CRT and IMRT. Int J Radiat Oncol Biol Phys 56:83–88. doi:10.1016/S0360-3016(03)00073-7
Duchesne GM (2001) Radiation for prostate cancer. Lancet Oncol 2:73–81. doi:10.1016/S1470-2045(00)00223-0
Mettler FA Jr, Huda W, Yoshizumi TT, Mahesh M (2008) Effective doses in radiology and diagnostic nuclear medicine: a catalog. Radiology 248:254–263. doi:10.1148/radiol.2481071451
Berrington de González A, Darby S (2004) Risk of cancer from diagnostic X-rays: estimates for the UK and 14 other countries. Lancet 31(363):345–351. doi:10.1016/S0140-6736(04)15433-0
Berrington de Gonzalez A, Curtis RE, Gilbert E, Berg CD, Smith SA, Stovall M, Ron E (2010) Second solid cancers after radiotherapy for breast cancer in SEER cancer registries. Br J Cancer 102:220–226. doi:10.1038/sj.bjc.6605435
Robinson BW, Musk AW, Lake RA (2005) Malignant mesothelioma. Lancet 366:397–408. doi:10.1016/S0140-6736(05)67025-0
Metz-Flamant C, Guseva Canu I, Laurier D (2011) Malignant pleural mesothelioma risk among nuclear workers: a review. J Radiol Prot 31:9–23. doi:10.1088/0952-4746/31/1/R01
Hawkins NM, Jhund PS, McMurray JJ, Capewell S (2012) Heart failure and socioeconomic status: accumulating evidence of inequality. Eur J Heart Fail 14:138–146. doi:10.1093/eurjhf/hfr168
Gershon AS, Dolmage TE, Stephenson A, Jackson B (2012) Chronic obstructive pulmonary disease and socioeconomic status: a systematic review. COPD 9:216–226. doi:10.3109/15412555.2011.648030
Currie GP, Watt SJ, Maskell NA (2009) An overview of how asbestos exposure affects the lung. BMJ 24(339):b3209. doi:10.1136/bmj.b3209
Hall WH, Jani AB, Ryu JK, Narayan S, Vijayakumar S (2005) The impact of age and comorbidity on survival outcomes and treatment patterns in prostate cancer. Prostate Cancer Prostatic Dis 8:22–30. doi:10.1038/sj.pcan.4500772
Abdollah F, Sun M, Schmitges J, Thuret R, Tian Z, Shariat SF, Briganti A, Jeldres C, Perrotte P, Montorsi F, Karakiewicz PI (2012) Competing-risks mortality after radiotherapy vs. observation for localized prostate cancer: a population-based study. Int J Radiat Oncol Biol Phys 84:95–103. doi:10.1016/j.ijrobp.2011.11.034
Cooperberg MR, Lubeck DP, Meng MV, Mehta SS, Carroll PR (2004) The changing face of low-risk prostate cancer: trends in clinical presentation and primary management. J Clin Oncol 22:2141–2149. doi:10.1200/JCO.2004.10.062
Lyratzopoulos G, Barbiere JM, Greenberg DC, Wright KA, Neal DE (2010) Population based time trends and socioeconomic variation in use of radiotherapy and radical surgery for prostate cancer in a UK region: continuous survey. BMJ 340:1928. doi:10.1136/bmj.c1928
McVey GP, McPhail S, Fowler S, McIntosh G, Gillatt D, Parker CC (2010) Initial management of low-risk localized prostate cancer in the UK: analysis of the British Association of Urological Surgeons Cancer Registry. BJU Int 106:1161–1164. doi:10.1111/j.1464-410X.2010.09288.x
Schymura MJ, Kahn AR, German RR, Hsieh MC, Cress RD, Finch JL, Fulton JP, Shen T, Stuckart E (2010) Factors associated with initial treatment and survival for clinically localized prostate cancer: results from the CDC-NPCR Patterns of Care Study (PoC1). BMC Cancer 10:152. doi:10.1186/1471-2407-10-152
Dahabreh IJ, Chung M, Balk EM, Yu WW, Mathew P, Lau J, Ip S (2012) Active surveillance in men with localized prostate cancer: a systematic review. Ann Intern Med 17(156):582–590. doi:10.1059/0003-4819-156-8-201204170-00397
Fraumeni JF Jr, Curtis RE, Edwards BK, Tucker MA (2006) Introduction. In: Curtis RE, Freedman DM, Ron E et al (eds) New malignancies among cancer survivors: SEER cancer registries, 1973–2000. National Cancer Institute, Bethesda, pp 1–7
Husain AN, Colby T, Ordonez N, Krausz T, Attanoos R, Beasley MB, Borczuk AC, Butnor K, Cagle PT, Chirieac LR, Churg A, Dacic S, Fraire A, Galateau-Salle F, Gibbs A, Gown A, Hammar S, Litzky L, Marchevsky AM, Nicholson A, Roggli V, Travis WD, Wick M (2012) Guidelines for pathologic diagnosis of malignant mesothelioma: 2012 Update of the consensus statement from the international mesothelioma interest group. Arch Pathol Lab Med. doi:10.5858/arpa.2012-0214-OA
Brenner DJ, Hall EJ, Curtis RE, Ron E (2005) Prostate radiotherapy is associated with second cancers in many organs, not just the colorectum. Gastroenterology 129:773–774. doi:10.1016/j.gastro.2005.06.045
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Farioli, A., Violante, F.S., Mattioli, S. et al. Risk of mesothelioma following external beam radiotherapy for prostate cancer: a cohort analysis of SEER database. Cancer Causes Control 24, 1535–1545 (2013). https://doi.org/10.1007/s10552-013-0230-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10552-013-0230-0