Abstract
Purpose
To access frequency and severity of adverse effects (AE) of non-hormonal drugs (NHD) for hot flashes in breast cancer survivors compared to controls and analyze adverse-effect risk by reviewing published randomized trials.
Methods
Cochrane Central Register for Controlled Trials, Embase, Medline, PsycINFO and PubMed databases were searched. Trials were included where participants were survivors of breast cancer suffering from hot flashes, treatment included self-administered venlafaxine, gabapentin or clonidine, and AE were reported. AE frequency and severity were graded. A meta-analysis of ten trials with sub-group analyses was conducted.
Results
Forty-nine studies were identified, and 12 were included. A total of 1467 participants experienced 772 adverse effects, 81 % (n = 627) in the treatment group and 19 % (n = 145) in the control group. Sixty-seven percent of AE was graded as mild and 33 % as moderate. The frequency of AE for NHD was overall significant compared to placebo. Sub-group analysis indicated that AE frequency and severity increased at higher doses of venlafaxine and gabapentin compared to placebo.
Conclusion
The odds for experiencing AE was significantly higher in patients randomized to high-dose NHD than those randomized to controls, including placebo, low-dose medication and acupuncture. These therapies should be considered as a potential treatment alternative.
Similar content being viewed by others
Introduction
Breast cancer is the second most common cancer in the world and the most frequent cancer among women. 1.67 million new cases were diagnosed in 2012 [1].
Treatment of breast cancer includes surgery, chemotherapy, radiation and endocrine therapy. Fifty percent of women diagnosed with breast cancer have a tumour that is oestrogen receptor positive, and consequently, they are offered hormone-suppression treatment lasting for at least five years [2]. Tamoxifen is an oestrogen receptor modulator which blocks the effect of oestrogen in breast tissue. It is indicated for use in premenopausal women and, as an initial treatment, in post-menopausal women. Aromatase inhibitors are recommended only for post-menopausal women, in whom the main source of oestrogen comes from the conversion of testosterone to estradiol, facilitated by the aromatase enzyme.
A common adverse effect of oestrogen-antagonist therapy is hot flashes. Up to 80 % of women medicated with tamoxifen suffer from hot flashes, 30 % of which rate them as severe [3, 4]. Severe hot flash problems can result in women stopping potentially lifesaving oestrogen-antagonist treatments; up to 25 % of women with breast cancer do not adhere to adjuvant oestrogen-antagonist therapy [5]. Consequently, better management of adverse effects including hot flashes is important for increasing compliance and achieving optimal results.
Self-administered treatments for hot flash problems such as drugs, creams or patches are the easiest and most practical therapy for most women. The most effective treatment is oestrogen therapy, but it is not recommended in women with breast cancer, and no safe conclusions regarding the use of progesterone are available [6]. Sixty percent of breast cancer tumours are oestrogen and/or progesterone receptor positive and therefore responsive to hormonal influence [7]. Contraindications surrounding hormonal therapies for the treatment of menopausal symptoms in breast cancer survivors have provoked increased use of non-hormonal drugs. Non-hormonal treatment includes therapies that do not affect oestrogen or progesterone production or action [8]. Self-administered therapies including anti-hypertensive medications, selective serotonin reuptake inhibitors (SSRI), selective norepinephrine reuptake inhibitors (SNRI), and anticonvulsant medicines have been studied for hot flash symptoms and increasingly used during the last decade. The most commonly used drugs in this category include venlafaxine, a selective serotonin reuptake inhibitor; the anticonvulsant gabapentin; and clonidine a centrally acting antiadrenergic agent, commonly used to control hypertension.
Randomized controlled trials (RCT) of drugs in these categories are limited; however, two systematic reviews have reported on the efficacy of these three drugs as a treatment for hot flashes in both breast cancer survivors and healthy menopausal women [8, 9]. Paroxetine and Fluoxetine, both being SSRIs, have also shown efficacy in the reduction of hot flashes [10–13]; however, these drugs interfere with the metabolization of tamoxifen to endoxifen [10, 14] and are therefore contraindicated in women using tamoxifen.
Various complementary and alternative therapies have been studied as a treatment for HF in breast cancer patients. Vitamin E has not demonstrated efficacy [8], while phytoestrogens possibly involve oestrogenic influence and are therefore not recommended for women with breast cancer [15]. Controversy around the safety of Cimicifuga Racemosa (Black Cohosh) as a treatment for menopausal symptoms exists because of its purported oestrogenic activity. A systematic review of 26 articles concluded that current evidence does not support an association between black cohosh and increased risk of breast cancer, and those conflicting but promising results for the reduction of HF in breast cancer patients warrant the need for further research [16]. Cognitive behavioural therapy trials [17, 18] and relaxation [19] have shown modest, short-term effect. Two trials investigating the effect of homoeopathy versus placebo [8], neither were RCT, found a statistically significant improvement in HF frequency for homoeopathy over placebo. Acupuncture was as effective as venlafaxine in a trial comparing these two interventions. However, 18 incidences of adverse effects were recorded in the venlafaxine group, whereas the acupuncture group experienced no adverse effects [20]. A systematic review of acupuncture to control hot flashes, which included 8 breast cancer studies (n = 474), concluded that the current level of evidence is insufficient to support the treatment of hot flashes [21].
The importance of this review
The efficacy and adverse-effect profiles of hot flash treatment vary in non-hormonal pharmacological interventions. Comparing studies of interventions in this category may provide an indication as to whether treatment effect outweighs adverse effects in breast cancer survivors. Potential information regarding the tolerability of each drug has direct clinical implications, affecting decision making and compliance.
Aims
The aims of this review are to
-
1.
systematically investigate how adverse effects of the three most commonly used non-hormonal drugs, to treat hot flashes in breast cancer patients, are reported in randomized controlled trials;
-
2.
classify adverse effects and drug-related aggravations according to the Common Terminology Criteria for Adverse Effects (CTCAE) [22] and
-
3.
perform a meta-analysis to evaluate the risk of adverse effects for patients pharmacologically managing their hot flashes with non-hormonal self-administered therapy, compared to different controls.
Terminology
If a substance is capable of producing a therapeutic effect, it can also produce harmful or unwanted effects. Terms used to describe such unwanted effects include side effect, adverse effect, adverse event, adverse reaction and toxic effect [23]. The term adverse effect used in this paper is defined by The European Medicines Agency [24] as any untoward medical occurrence in a patient or clinical trial subject administered a medical product. This term encompasses all unwanted effects, without making assumptions about their mechanism [25].
Methods
Search methods for identification of studies
The focus question was: Are the most commonly used non-hormonal drugs for hot flashes in breast cancer patients associated with adverse effects? The four elements from PICO were used when searching for relevant articles:
-
1.
Population: Patients with breast cancer, suffering from hot flashes.
-
2.
Intervention: Non-hormonal self-administered pharmacological therapies, including venlafaxine, gabapentin and clonidine.
-
3.
Comparison: Placebo, other non-hormonal drugs, conventional medical therapies, CAM, waiting list and usual care.
-
4.
Outcome: Adverse effects, adverse events, adverse reactions, tolerability, side effects and toxicity.
The following electronic databases were searched with no language, publication, or time restrictions: Cochrane Central Register for Controlled Trials (Central) in the Cochrane library, Embase, Medline, PsycINFO and PubMed.
Titles and abstracts were identified through the search strategy. If no abstract was available, the full text paper was obtained for inspection. Both authors did the searches, read the articles and extracted the data (search strings are attached in the appendix). Grey literature was searched in order to find possibly missed articles through electronic searches. References of all retrieved articles and systematic reviews were searched [8, 9, 26–28]. Depending on the database, various combinations of MESH terms and keywords were used. MESH terms included breast neoplasms, breast cancer, hot flashes, clonidine, adverse effect, adverse drug reaction reporting systems. The following keywords were applied: breast cancer, hot flash, hot flush, vasomotor symptom, clonidine, venlafaxine, gabapentin, adverse effect, adverse event and side-effect.
Inclusion comprised randomized controlled trials that reported adverse effects of treatment. Both parallel group design and cross-over studies were included. Data from cross-over studies were included from both treatment periods, since all cross-over studies specified that there was no cross-over effect.
Data were extracted to give information on the total number of adverse effects and number of patients experiencing the adverse effects. Severity of adverse effects was assessed using the CTCAE grading system and was entirely dependent on the information provided in the articles. The system grades adverse effects from 1 to 5, where 1 indicates mild symptoms, 2 moderate symptoms, 3 severe symptoms, 4 life threatening and 5 fatal symptoms. When summarizing the data, the total number of adverse effects was counted, regardless of the number of participants experiencing them. Both authors categorized and graded the data. Lack of consensus was settled by discussion.
A methodological assessment including risk of bias was made by both authors using criteria from the Cochrane Handbook of Systematic Reviews and Interventions [29]. The trials were rated as follows:
A grading of “A” indicates a RCT of high quality with low risk of bias with adequate measures to conceal allocation, detailed randomization description and implementation of the intention to treat principle.
Grade “B” was used when method of allocation concealment was not described, or was unclear, creating a moderate risk of bias.
A grade “C” was used when the method of allocation was not concealed; such trials were excluded because of high risk of bias.
Extracted data included number of patients randomized to each group, number of dropouts, use of power calculation, whether the intention to treat principle was followed, intervention (including dose), duration of intervention, main findings and funding. The authors of retrieved articles were contacted when in doubt of or there is a lack of information in the publications (Table 1).
Meta-analysis
Study populations were divided into groups experiencing adverse effects versus those with no adverse effects in both treatment and control groups. Homogenous study designs including participants, interventions, control groups and outcome measures were combined and a meta-analysis performed; P < 0.10 defined significant heterogeneity. Odds ratios and 95 % confidence intervals were calculated from the number of patients experiencing adverse effects in each group based on the total number of patients randomized to either treatment or control group. Studies with no adverse effects either in one or both groups were given an added continuity correction of 0.5 in order to estimate a valid approximation of odds ratio [30]. Data regarding the adverse effect in a trial carried out by Boekhout, which compared venlafaxine and clonidine to placebo, were found to be identical for both the venlafaxine and clonidine groups [31]. These data were included only once in the meta-analysis to avoid overrepresentation of adverse effects in the intervention group. Three studies comparing different drug dosages to placebo were divided according to high and low dosage in the meta-analysis [32–34]. Based on the total number of participants randomized to the treatment or control group, odds ratios and 95 % confidence intervals were calculated from the number of patients experiencing adverse effects in each group. To perform a meta-analysis, data were entered directly from the datasheets into Review Manager 5 computer program [35].
Results
Outcome of the literature searches
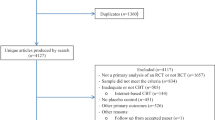
A total of 49 articles were identified. They were initially examined on the basis of titles and abstracts; 37 were excluded from further examination due to the following: 30 did not meet inclusion criteria and seven were multiple article registrations in databases. A total of 12 articles were included in this review (Fig. 1).
The control intervention was clonidine in three studies [31, 37, 39] and placebo in five studies. These five studies compared venlafaxine, or clonidine, or gabapentin to placebo [32–34, 37, 38]. Two of these studies examined venlafaxine at two [32] and three [33] different doses and one study examined gabapentin at two different doses [34]. Three studies compared gabapentin to other therapies: vitamin E [39], hypnotherapy [40] and electro-acupuncture [41], and one compared venlafaxine to acupuncture [20].
Methodological assessment as described in the Cochrane handbook was used to rate the included trials: All were classified as high quality (A), apart from three RCTs in which risk of bias was increased by providers and participants not being blinded [20, 39] and one where no blinding was used and the sample size was small [40].
Five studies included more than one active treatment arm [31–34, 41]. Four studies had a cross-over design [32, 36, 38, 42]. Number of participants ranged from a minimum of 27 to a maximum of 420. The duration of the studies ranged from 4 to 24 weeks.
Types of adverse effects were reported in all the included studies. Number of patients suffering from adverse effects and number of adverse effects were reported in all but two studies [32, 38] where specific adverse effects were compared to placebo and reported as p values. We tried to contact the authors of these two studies in order to gain access to more comparable data. We were not able to get in touch with Goldberg; Carpenter kindly provided more data, but the actual numbers concerning adverse effects were not available. These two studies were consequently excluded from the meta-analysis. One study presented data on adverse effects only if these were the reason for dropping out, possibly causing an underestimation of the number of adverse effects [34]. A total of 1467 participants experienced 772 adverse effects. Of these, 81 % (n = 627) were in the treatment group and 19 % (n = 145) were in the control group. Adverse effects included appetite disorder, nausea, dry mouth, fatigue, dizziness, headache, difficulty sleeping, anxiety, memory problems, sweating, constipation, double vision and increased blood pressure.
Sixty-seven percent of the adverse effects were graded as CTCAE I (n = 515) and 33 % were graded as CTCAE II (n = 257) (Table 2). Adverse effects causing participants to dropout were classified as CTCAE grade II.
Whether dropping-out in the included studies was due to adverse effects was reported in all but four studies [33, 34, 38, 41]. In the three studies comparing venlafaxine and clonidine, the number of dropouts due to adverse effects were fourteen and five [36], six and four [42] and six and two [31], respectively, totalling 26 in the venlafaxine groups versus 11 in the clonidine groups. Gabapentin was compared to placebo [34], hypnotherapy [40] and vitamin E [39], where sixteen, three and seventeen women, respectively, dropped out of the gabapentin groups due to adverse effects; there were no dropouts in the second arms. Venlafaxine was compared to placebo [32], where the number of dropouts were 3 versus 1, and acupuncture [20], where the only dropouts due to adverse effects were 3 women in the venlafaxine group.
Meta-analyses
Adverse effects’ data from 10 RCTs were included in the meta-analysis with a total of 1,428 subjects.
Non-hormonal medication versus overall control
An overall comparison was made between non-hormonal medication and control. Ten trials had 13 different outcomes due to low and high drug doses in the same trials. A significant difference was found between non-hormonal medication and control, with OR of 1.67, 95 % CI of 1.31–2.13 and I 2 of 85 % (P < 0.0001).
Different sub-group meta-analyses according to the categories of controls were performed, and are presented below.
Non-hormonal medication versus placebo
A comparison was made between non-hormonal medication and placebo. Two trials (259 participants) made this comparison, and a statistically significant difference was found between non-hormonal medication and placebo, with OR of 1.51, 95 % CI of 1.16–1.98 and I 2 of 0 % (P = 0.002).
High-dose non-hormonal medication versus placebo
There was no statistically significant difference between high-dose non-hormonal medication and placebo in a meta-analysis of two trials (n = 450) for three different combined outcomes, with OR of 2.96, 95 % CI of 0.97–9.05 I 2 and 93 % (P = 0.06).
Low-dose non-hormonal medication versus placebo
A comparison was made between low-dose non-hormonal medication and placebo. Two trials (345 participants) made this comparison, and no statistically significant difference was found between the groups (OR 1.53, 95 % CI 0.62–3.77, I 2 = 87 %, P = 0.36).
Non-hormonal medication versus non-hormonal medication
There was a significant difference between non-hormonal medication (venlafaxine) and non-hormonal medication (clonidine) in a meta-analysis of two trials, with OR of 1.44, 95 % CI of 1.00–2.08 and I 2 of 45 % (P = 0.05).
Non-hormonal medication versus acupuncture
A comparison was made between non-hormonal medication and acupuncture. Two trials (108 participants) made this comparison; a significant difference was found between the groups in favour of acupuncture, with OR of 1.75, 95 % CI of 01.09–2.75 and I 2 of 0 % (P = 0.02).
Non-hormonal medication versus other therapy
There was no statistically significant difference between non-hormonal medication and other therapies in a meta-analysis of two trials, with OR of 1.34, 95 % CI of 0.74–2.45 and I 2 of 34 % (P = 0.33).
Discussion
This meta-analysis demonstrated that the odds for experiencing adverse effects was significantly higher in patients randomized to non-hormonal medication than for patients randomized to controls, such as placebo and acupuncture. High-dose non-hormonal medication (venlafaxine and gabapentin) provoked an increased number of adverse effects compared to low-dose medication. This may suggest that low-dose non-hormonal medication is a good alternative for breast cancer survivors with hot flashes, providing sufficient reduction in frequency and intensity of hot flashes. Rada et al. [8] in their systematic review report that non-hormonal therapies have a mild to moderate effect in reducing frequency and intensity of hot flashes in women with a history of breast cancer. This result was based on nine different studies evaluating the effect of SSRIs (n = 6), clonidine (n = 2) and gabapentin (n = 1).
Acupuncture has few adverse effects compared to non-hormonal medication and should be considered as a potential treatment alternative if efficacy can be confirmed in future studies. Four systematic reviews evaluating acupuncture for hot flashes in breast cancer survivors included six [44], seven [43], eight [21] and twelve [45] RCT’s respectively. Overall, authors concluded that acupuncture effectively reduced hot flashes, but was not statistically significant compared to sham; and that there is currently insufficient evidence to either support or refute acupuncture for this patient category.
Twelve trials were identified for this systematic review, and ten of these were included in the meta-analysis. We pooled results in an attempt to give an overall comparison of non-hormonal medication versus control; six different sub-group analyses were done. However, only two trials made up each group, thereby only demonstrating tendencies.
Study strengths and limitations
As far as we know, this is the first systematic review and meta-analysis to examine adverse effects of non-hormonal medications for hot flashes in breast cancer survivors, as the primary outcome measure. The included studies were of high methodological quality and with reduced risk of bias, thereby providing reliable results. Heterogeneity is always an important consideration when compared to RCTs, and the forest plot showed strong study similarities.
Two-thirds of adverse effects reported in this review were classified as grade I and a third as grade II. The CTCAE grading of adverse effects was solely based on information provided by the articles included in this review, and should be considered as an approximation of adverse effect severity. Inconsistent use of safety terminology made it difficult to categorize and evaluate the data; the CTCAE grading system was not consistently used.
Three different non-hormonal medications were assessed and compared to different control groups. This was a limiting factor in the meta-analysis. To reduce the risk of inflating the size of the pooled treatment effect, zero-cell counts were included [46]. A continuity correction of 0.5 was used for studies with zero-cell counts, in order to provide a conservative approximation of adverse event risk [47].
Six studies included in the meta-analysis had active controls, including other non-hormonal medicines, acupuncture and other therapies, possibly inflating adverse-effect frequency outcomes; however, the forest plot (Fig. 2) does not indicate such influence when studies with active controls are compared to those with passive controls.
Other elements of conceivable bias include possible under-reporting of adverse effects by participants motivated to experience treatment effect, simply due to being included in a clinical trial. Publication bias is also a consideration; clinical trials demonstrating a statistically significant treatment effect compared to control are more likely to be published [48].
Search strategy for this review included five search engines, and more RCTs may have been identified if more search engines had been added. However, we also identified a systematic review focusing on active interventions for hot flash symptoms in breast cancer patients [8] and 4 reviews focusing on a combination of post-menopausal women and breast cancer survivors [9, 26–28]. Examination of the full texts and reference lists of these reviews did not provide any additional RCTs for this meta-analysis.
Other studies
To our knowledge, only one systematic review evaluating non-hormonal therapies for hot flashes in women with a history of breast cancer has been published, and the focus was on treatment efficacy [8]. We could not find any systematic reviews that examined adverse effects due to non-hormonal drugs as a primary outcome in this patient category. Rada and colleagues reported evidence supporting the use of clonidine, gabapentin and SSRIs/SNRIs for hot flash symptoms in breast cancer survivors. The authors commented that adverse effects were inconsistently reported. 16 studies were included, of which 10 were pharmacological studies and 6 non-pharmacological studies. They confirmed our findings that adverse effects increase when higher doses of gabapentin and venlafaxine were used. They also suggested that adverse effects may outweigh benefit in clonidine.
Another systematic review of 13 randomized trials comparing active interventions for hot flash problems in women with and without breast cancer [26] did not agree with our findings of dose-related increased frequency of adverse effects. The authors reported that high doses of venlafaxine (75 mg/day) and gabapentin (900 mg/day) appeared to improve hot flash symptoms to a greater extent compared to lower doses, without incurring more adverse effects. Since the population did not only include breast cancer patients, the results make comparison with our study difficult.
Implications
Despite these limitations, the sub-group analyses provided information relevant for clinical practice, including the relationship between drug dosage and adverse effects, drug comparisons in relation to adverse effects and the possible role of acupuncture as a treatment for hot flashes if efficacy can be confirmed. Further research is indicated to investigate these findings with focus on efficacy versus adverse effects; also the effect of combined therapies should be considered with a view to increasing the compliancy of oestrogen-antagonist medication.
Conclusion
The odds for experiencing adverse effects was statistically significantly higher in patients randomized to high-dose non-hormonal medication than for patients randomized to controls, such as placebo, low-dose medication and acupuncture. Consequently, these therapies should be considered as a potential treatment alternative if efficacy for hot flushes can be confirmed.
References
World Health Organization. IARC, International agency for research on cancer WHO. www.who.int
Perry N, Broeders M, de Wolf C, Törnberg S, Holland R, von Karsa L (2008) European guidelines for quality assurance in breast cancer screening and diagnosis. Fourth edition—summary document. Ann Oncol 19(4):614–622
Day R, Ganz PA, Costantino JP, Cronin WM, Wickerham DL, Fisher B (1999) Health-related quality of life and tamoxifen in breast cancer prevention: a report from the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Clin Oncol 17(9):2659–2669
Kligman L, Younus J (2010) Management of hot flashes in women with breast cancer. Curr Oncol 17(1):81–86
Partridge A, Burnstein HJ, Winer EP (2001) Side effects of chemotherapy and combined chemohormonal therapy in women with early-stage breast cancer. J Natl Cancer Inst Monogr 30:135–142
Loprinzi CL, Barton DL, Qin R (2011) Nonestrogenic management of hot flashes. J Clin Oncol 29(29):3842–3846
Allegra JC, Lippman ME (1980) Estrogen receptor status and the disease-free interval in breast cancer. Recent Results Cancer Res 71:20–25
Rada G, Capurro D, Pantoja T, Corbalán J, Moreno G, Letelier LM, Vera C (2010) Non-hormonal interventions for hot flushes in women with a history of breast cancer. Cochrane Database Syst Rev. doi:10.1002/14651858.CD004923
Nelson HD, Vesco KK, Haney E, Fu R, Nedrow A, Miller J, Nicolaidis C, Walker M, Humphrey L (2006) Nonhormonal therapies for menopausal hot flashes: systematic review and meta-analysis. JAMA 295(17):2057–2071
Stearns V, Beebe KL, Iyengar M, Dube E (2003) Paroxetine controlled release in the treatment of menopausal hot flashes: a randomized controlled trial. JAMA 289(21):2827–2834
Stearns V, Slack R, Greep N, Henry-Tilman R, Osborne M, Bunnell C, Ullmer L, Gallagher A, Cullen J, Gehan E, Hayes DF, Isaacs C (2005) Paroxetine is an effective treatment for hot flashes: results from a prospective randomized clinical trial. J Clin Oncol 23(33):8549
Loprinzi CL, Sloan JA, Perez EA, Quella SK, Stella PJ, Mailliard JA, Halyard MY, Pruthi S, Novotny PJ, Rummans TA (2002) Phase III evaluation of fluoxetine for treatment of hot flashes. J Clin Oncol 20(6):1578–1583
Suvanto-Luukkonen E, Koivunen R, Sundstrom H, Bloigu R, Karjalainen E, Häivä-Mällinen L, Tapanainen JS (2005) Citalopram and fluoxetine in the treatment of post-menopausal symptoms: a prospective, randomized 9-month, placebo-controlled, double-blind study. Menopause. 12(1):18–26
Wu AH, Stanczyk FZ, Martinez C, Tseng CC, Hendrich S, Murphy P, Chaikittisilpa DO, Stram S, Pike MC (2005) A controlled 2-mo dietary fat reduction and soy food supplementation study in postmenopausal women. Am J Clin Nutr 81(5):1133–1141
Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SA, Howard BV, Johnson KC, Kotchen JM, Ockene J (2002) Writing Group for the Women’s Health Initiative Investigators Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. JAMA 288(3):321–333
Fritz H, Seely D, McGowan J, Skidmore B, Fernandes R, Kennedy DA, Cooley K, Wong R, Sagar S, Balneaves LG, Fergusson D (2014) Black cohosh and breast cancer: a systematic review. Integr Cancer Ther 13(1):12–29
Mann E, Smith MJ, Hellier J, Balabanovic JA, Hamed H, Grunfeld EA, Hunter MS (2012) Cognitive behavioural treatment for women who have menopausal symptoms after breast cancer treatment (MENOS 1): a randomized controlled trial. Lancet Oncology 13(3):309–318
Duijts SF, van Beurden M, Oldenburg HS, Hunter MS, Kieffer JM, Stuiver MM, Gerritsma MA, Menke-Pluymers MB, Plaisier PW, Rijna H, Lopes Cardozo AM, Timmers G, van der Meij S, van der Veen H, Bijker N, de Widt-Levert LM, Geenen MM, Heuff G, van Dulken EJ, Boven E, Aaronson NK (2012) Efficacy of cognitive behavioural therapy and physical exercise in alleviating treatment induced menopausal symptoms in patients with breast cancer: results of a randomized, controlled multicenter trial. J Clin Oncol 30(33):4124–4133
Fenlon DR, Corner JL, Haviland JS (2008) A randomized controlled trial of relaxation training to reduce hot flashes in women with primary breast cancer. J Pain Symptom Manage 35(4):397–405
Walker EM, Rodriguez AI, Kohn B, Ball RM, Pegg J, Pocock JR, Nunez R, Peterson E, Jakary S, Levine RA (2010) Acupuncture versus venlafaxine for the management of vasomotor symptoms in patients with hormone receptor-positive breast cancer: a randomized controlled trial. J Clin Oncol 28(4):634–640
Garcia MK, McQuade J, Haddad R et al (2013) Systematic review of acupuncture in cancer care: a synthesis of the evidence. J Clin Oncol 31(7):952–960
U.S. Department of Health and Human Services (2010) Common terminology criteria for adverse events v 4.0 (CTCAE). National Institutes of Health, National Cancer Care Institute. http://evs.nci.nih.gov/
Nebeker JR, Barach P, Samore MH (2004) Clarifying adverse drug events: a clinician’s guide to terminology, documentation, and reporting. Ann Intern Med 140(10):795–802
European Medicines Agency. (2002) ICH Topic E 6 (R1) guideline for good clinical practice. London: European Medicines Agency, Contract No: E 6 (R1). http://www.ema.europa.eu/
Edwards IR, Aronson JK (2000) Adverse drug reactions: definitions, diagnosis, and management. The Lancet. 356(9237):1255–1259
Johns C, Seav SM, Dominick SA, Gorman JR, Li H, Natarajan L, Mao JJ, Irene SuH (2016) Informing hot flash treatment decisions for breast cancer survivors: a systematic review of randomized trial comparing active interventions. Breast Cancer Res Treat 156(3):415–426
Cheema D, Coomarasamy A, El-Toukhy T (2007) Non-hormonal therapy of post-menopausal vasomotor symptoms: a structured evidence-based review. Arch Gynecol Obstet 276(5):463–469
Bordeleau L, Pritchard K, Goodwin P, Loprinzi C (2007) Therapeutic options for the management of hot flashes in breast cancer survivors: an evidence-based review. Clin Ther 29(2):230–241
Higgins JPT. (2011) Cochrane handbook for systematic reviews of interventions 5.1.0. Green S, editor. Chichester U.K.: Wiley & Sons, Ltd, New York
Bhaumik DK, Amatya A, Normand SL, Greenhouse J, Kaizar E, Neelon B, Gibbons RD (2012) Meta-analysis of rare binary event data. J Am Stat Assoc 107(498):555–567
Boekhout AH, Vincent AD, Dalesio OB, Bosch van den J, Foekema-Töns JH, Adriaansz S, Sprangers S, Nuijen B, Beijnen JH, Schellens JH (2011) Management of hot flashes in patients who have breast cancer with venlafaxine and clonidine: a randomized, double-blind, placebo-controlled trial. J Clin Oncol 10(29):3862–3868
Carpenter JS, Storniolo AM, Johns S, Monahan PO, Azzouz F, Elam JL, Johnson CS, Shelton RC (2007) A Randomized, double-blind, placebo-controlled crossover trials of venlafaxine for hot flashes after breast cancer. Oncologist 12(1):124–135
Loprinzi CL, Kugler JW, Sloan JA, Mailliard JA, LaVasseur BI, Barton DL, Novotny PJ, Dakhil SR, Rodger K, Rummans TA, Christensen BJ (2000) Venlafaxine in management of hot flashes in survivors of breast cancer: a randomised controlled trial. Lancet 356(9247):2059–2063
Pandya KJ, Morrow GR, Roscoe JA, Zhao H, Hickok JT, Pajon E, Sweeney TJ, Banerjee TK, Flynn PJ (2005) Gabapentin for hot flashes in 420 women with breast cancer: a randomised double-blind placebo-controlled trial. Lancet 366(9488):818–824
Cochrane informatics and knowledge management system (2014) Review Manager 5 (RevMan). http://tech.cochrane.org/revman
Buijs C, Mom CH, Willemse PH, Marike Boezen H, Maurer JM, Wymenga AN, de Jong RS, Nieboer P, de Vries EG, Mourits MJ (2009) Venlafaxine versus clonidine for the treatment of hot flashes in breast cancer patients: a double-blind, randomized cross-over study. Breast Cancer Res Treat 115(3):573–580
Pandya KJ, Raubertas RF, Hynes HE, Rosenbluth RJ, Kirshner JJ, Pierce HI, Dragalin V, Morrow GR (2000) Oral clonidine in postmenopausal patients with breast cancer experiencing tamoxifen-induced hot flashes: a University of Rochester Cancer Center Community Clinical Oncology Program Study. Ann Intern Med 132(10):788–793
Goldberg RM, Loprinzi CL, O’Fallon JR, Patel S, Lee R, Yang P, Palmer JL, Cohen L (1994) Transdermal clonidine for ameliorating tamoxifen-induced hot flashes. J Clin Oncol 12(1):155–158
Biglia N, Sgandurra P, Peano E, Marenco D, Moggio G, Bounous V, Tomasi Cont N, Ponzone R, Sismondi P (2009) Non-hormonal treatment of hot flushes in breast cancer survivors: gabapentin versus vitamin E. Climacteric 12(4):310–318
Maclaughlan DS, Salzillo S, Bowe P, Scuncio S, Malit B, Raker C, Gass J, Granai C, Dizon D (2013) Randomized controlled trial comparing hypnotherapy versus gabapentin for the treatment of hot flashes in breast cancer survivors: a pilot study. BMJ Open. doi:10.1136/bmjopen-2013-003138
Mao JJ, Bowman MA, Xie SX, Bruner D, DeMichele A, Farrar JT (2015) Electro-acupuncture versus gabapentin for hot flashes among breast cancer survivors: a randomized placebo-controlled trial. J Clin Oncol 33(31):3615–3620
Loibl S, Schwedler K, von Minckwitz G, Strohmeier R, Mehta KM, Kaufmann M (2007) Venlafaxine is superior to clonidine as treatment of hot flashes in breast cancer patients–a double-blind, randomized study. Ann Oncol 18(4):689–693
Chiu HY, Shyu YK, Chang PC, Tsai PS (2016) Effects of Acupuncture on Menopause-Related Symptoms in Breast Cancer Survivors: a Meta-analysis of Randomized Controlled Trials. Cancer Nurs 39(3):228–237
Lee MS, Kim KH, Choi SM, Ernst E (2009) Acupuncture for treating hot flashes in breast cancer patients: a systematic review. Breast Cancer Res Treat 115(3):497–503
Dos Santos S, Hill N, Morgan A, Smith J, Thai C, Cheifetz O (2010) Acupuncture for treating common side effects associated with breast cancer treatment: a systematic review. Medical Acupuncture 22(2):81–97
Friedrich JO, Adhikari NK, Beyene J (2007) Inclusion of zero total event trials in meta-analyses maintains analytic consistency and incorporates all available data. BMC Med Res Methodol 23:5–7
Sweeting MJ, SuttonAJ Lambert PC (2004) What to add to nothing? Use and avoidance of continuity corrections in meta-analysis of sparse data. Stat Med 23(9):1351–1375
Vickers A, Goyal N, Harland R, Rees R (1998) Do certain countries produce only positive results? A systematic review of controlled trials. Control Clin Trials 19(2):159–166
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors Jill Hervik and Trine Stub declare no conflict of interest.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Hervik, J.B., Stub, T. Adverse effects of non-hormonal pharmacological interventions in breast cancer survivors, suffering from hot flashes: A systematic review and meta-analysis. Breast Cancer Res Treat 160, 223–236 (2016). https://doi.org/10.1007/s10549-016-4002-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-016-4002-x