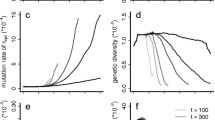
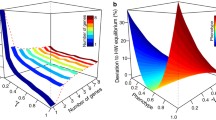
Abstract
The role of adaptation in determining invasion success has been acknowledged recently, notably through the accumulation of case studies of rapid evolution during bioinvasions. Despite this growing body of empirical evidence, there is still a need to develop the theoretical background of invasions with adaptation. Specifically, the impact of mating system on the dynamics of adaptation during invasion of a new environment remains only partially understood. Here, we analyze a simulation demo-genetic model of bioinvasion accounting for partial asexuality rates. We simulate two levels of recurrent immigration from a source population at mutation–drift–selection equilibrium to a new empty environment with a different adaptive landscape (black-hole sink). Adaptation relies on a quantitative trait coded explicitly by 10 loci under mutation, selection and genetic drift. Using this model, we confirm previous results on the positive effects on invasiveness of migration, mutation and similarity of local phenotypic optima. We further show how the invasion dynamics of the introduced population is affected by the rate of asexuality. Purely asexual species have lower invasion success in terms of probability and time to invasion than species with other mating systems. Among species with mixed mating systems, the greatest invasiveness is observed for species with high asexual rates. We suggest that this pattern is due to inflated genetic variance in the source population through the Hill-Robertson effect (i.e., clonal interference). An interesting consequence is that species with the highest genetic load in their source environment have greatest invasiveness in the new environment.





Similar content being viewed by others
References
Amsellem L, Noyer J-L, Hossaert-McKey M (2001) Evidence for a switch in the reproductive biology of Rubus alceifolius (Rosaceae) towards apomixis, between its native range and its area of introduction. Am J Bot 88:2243–2251
Baker HG (1955) Self-compatibility and establishment after ‘long-distance’ dispersal. Evolution 9:347–349
Barrett SCH (2011) Why reproductive systems matter for the invasion biology of plants. In: Richardson DM (ed) Fifty years of invasion ecology: the legacy of Charles Elton, 1st edn. Blackwell Publishing Ltd, Hoboken, pp 195–210
Barrett RDH, Schluter D (2008) Adaptation from standing genetic variation. Trends Ecol Evol 23:38–44
Barrett SCH, Colautti RI, Eckert CG (2008) Plant reproductive systems and evolution during biological invasion. Mol Ecol 17:373–383
Behrman KD, Kirkpatrick M (2011) Species range expansion by beneficial mutations. J Evol Biol 24:665–675
Bridle JR, Polechová J, Kawata M, Butlin RK (2010) Why is adaptation prevented at ecological margins? New insights from individual-based simulations. Ecol Lett 13:485–494
Brown AHD, Burdon JJ (1987) Mating systems and colonizing success in plants. In: Cary AJ, Crawley MJ, Edwards PJ (eds) Colonization, succession and stability. Blackwell Scientific Publications, Oxford, pp 115–131
Bürger R (1989) Linkage and the maintenance of heritable variation by mutation–selection balance. Genetics 121:175–184
Bürger R, Lynch M (1995) Evolution and extinction in a changing environment: a quantitative-genetic analysis. Evolution 49:151–163
Bürger R, Lynch M (1997) Adaptation and extinction in changing environments. Genetics 83:209–239
Burns JH, Ashman TL, Steets JA et al (2011) A phylogenetically controlled analysis of the roles of reproductive traits in plant invasions. Oecologia 166:1009–1017
Burt A (2000) Perspective: sex, recombination, and the efficacy of selection—was Weismann right? Evolution 54:337–351
Charlesworth D, Morgan MT, Charlesworth B (1993) Mutation accumulation in finite outbreeding and inbreeding populations. Genet Res 61:39–56
Cox GW (2004) Alien species and evolution. Island Press, Washington, D C
D’Souza TG, Michiels NK (2010) The costs and benefits of occasional sex: theoretical predictions and a case study. J Hered 101(Suppl):S34–S41
Desprez-Loustau M-L, Robin C, Buée M et al (2007) The fungal dimension of biological invasions. Trends Ecol Evol 22:472–480
Dlugosch KM, Parker IM (2008) Founding events in species invasions: genetic variation, adaptive evolution, and the role of multiple introductions. Mol Ecol 17:431–449
Ellstrand NC, Schierenbeck KA (2000) Hybridization as a stimulus for the evolution of invasiveness in plants? Proc Natl Acad Sci USA 97:7043–7050
Facon B, Pointier J-P, Jarne P et al (2008) High genetic variance in life-history strategies within invasive populations by way of multiple introductions. Curr Biol 18:363–367
Felsenstein J (1974) The evolutionary advantage of recombination. Genetics 78:737–756
Gomulkiewicz R, Holt RD, Barfield M (1999) The effects of density dependence and immigration on local adaptation and niche evolution in a black-hole sink environment. Theor Popul Biol 55:283–296
Green RF, Noakes DLG (1995) Is a little bit of sex as good as a lot? J Theor Biol 174:87–96
Grether G (2005) Environmental change, phenotypic plasticity, and genetic compensation. Am Nat 166:E115–E123
Hayes K, Barry S (2008) Are there any consistent predictors of invasion success? Biol Invasions 10:483–506
Hedrick PW, Whittam TS (1989) Sex in diploids. Nature 342:231
Hill WG, Robertson A (1966) Linkage disequilibrium in finite populations. Theor Appl Genet 38:226–231
Holt RD (1983) Models for peripheral populations: the role of immigration. In: Freedman HI, Strobeck C (eds) Lecture notes in biomathematics. Springer, Berlin, pp 25–32
Holt RD (1996) Demographic constraints in evolution: towards unifying the evolutionary theories of senescence and niche conservatism. Evol Ecol 10:1–11
Holt RD, Gaines M (1992) The analysis of adaptation in heterogeneous landscapes: implications for the evolution of fundamental niches. Evol Ecol 6:433–447
Holt RD, Gomulkiewicz R (1997a) How does immigration influence local adaptation? A reexamination of a familiar paradigm. Am Nat 149:563–572
Holt RD, Gomulkiewicz R (1997b) The evolution of species’ niches: a population dynamic perspective. In: Othmer H, Adler F, Lewis M, Dallon J (eds) Case studies in mathematical modeling: ecology, physiology, and cell biology. Prentice-Hall, New Jersey, pp 25–50
Holt RD, Gomulkiewicz R, Barfield M (2003) The phenomenology of niche evolution via quantitative traits in a “black-hole” sink. Proc R Soc Lond B Biol Sci 270:215–224
Holt RD, Knight TM, Barfield M (2004) Allee effects, immigration, and the evolution of species’ niches. Am Nat 163:253–262
Holt RD, Barfield M, Gomulkiewicz R (2005) Theories of Niche conservatism and evolution. Could exotic species be potential tests? In: Sax DF, Stachowicz JJ, Gaines SD (eds) Species invasions. Insights into ecology, evolution, and biogeography. Sinauer, Sunderland, pp 269–279
Hufbauer RA, Facon B, Ravigné V et al (2012) Anthropogenically induced adaptation to invade (AIAI): contemporary adaptation to human-altered habitats within the native range can promote invasions. Evol Appl 5:89–101
Kanarek AR, Webb CT (2010) Allee effects, adaptive evolution, and invasion success. Evol Appl 3:122–135
Kawecki TJ (1995) Demography of source-sink populations and the evolution of ecological niches. Evol Ecol 9:38–44
Kawecki TJ (2000) Adaptation to marginal habitats: contrasting influence of the dispersal rate on the fate of alleles with small and large effects. Proc R Soc Lond B Biol Sci 267:1315–1320
Kawecki TJ (2008) Adaptation to marginal habitats. Annu Rev Ecol Evol Syst 39:321–342
Keane RM, Crawley MJ (2002) Exotic plant invasions and the enemy release hypothesis. Trends Ecol Evol 17:164–170
Kimbrell T, Holt RD (2007) Canalization breakdown and evolution in a source-sink system. Am Nat 169:370–382
Kirkpatrick M, Barton NH (1997) Evolution of a species’ range. Am Nat 150:1–23
Kolar CS, Lodge DM (2001) Progress in invasion biology: predicting invaders. Trends Ecol Evol 16:199–204
Lambrinos JG (2004) How interactions between ecology and evolution influence contemporary invasion dynamics. Ecology 85:2061–2070
Larkin DJ (2012) Lengths and correlates of lag phases in upper-Midwest plant invasions. Biol Invasions 14:827–838
Lee CE (2002) Evolutionary genetics of invasive species. Trends Ecol Evol 17:386–391
Liu H, Stiling P (2006) Testing the enemy release hypothesis: a review and meta-analysis. Biol Invasions 8:1535–1545
Lloret F, Medail F, Brundu G et al (2005) Species attributes and invasion success by alien plants on Mediterranean islands. J Ecol 93:512–520
Lynch M, Gabriel W (1983) Phenotypic evolution and parthenogenesis. Am Nat 122:745–764
Martin G, Otto SP, Lenormand T (2006) Selection for recombination in structured populations. Genetics 172:593–609
McDonald BA, Linde CC (2002) Pathogen population genetics, evolutionary potential, and durable resistance. Annu Rev Phytopathol 40:349–379
Muller-Scharer H, Schaffner U, Steinger T (2004) Evolution in invasive plants: implications for biological control. Trends Ecol Evol 19:417–422
Neuenschwander S, Hospital F, Guillaume F, Goudet J (2008) quantiNemo: an individual-based program to simulate quantitative traits with explicit genetic architecture in a dynamic metapopulation. Bioinformatics 24:1552–1553
Otto SP, Lenormand T (2002) Resolving the paradox of sex and recombination. Nat Rev Genet 3:252–261
Pannell JR, Barrett SCH (1998) Baker’s law revisited: reproductive assurance in a metapopulation. Evolution 52:657–668
Pannell JR, Dorken ME (2006) Colonisation as a common denominator in plant metapopulations and range expansions: effects on genetic diversity and sexual systems. Landsc Ecol 21:837–848
Parker IM, Gilbert GS (2004) The evolutionary ecology of novel plant–pathogen interactions. Annu Rev Ecol Evol Syst 35:675–700
Peck JR, Yearsley JM, Waxman D (1998) Explaining the geographic distributions of sexual and asexual populations. Nature 391:889–892
Philibert A, Desprez-Loustau M-L, Fabre B et al (2011) Predicting invasion success of forest pathogenic fungi from species traits. J Appl Ecol 48:1381–1390
Pigliucci M, Murren CJ (2003) Perspective: genetic assimilation and a possible evolutionary paradox: can macroevolution sometimes be so fast as to pass us by? Evolution 57:1455–1464
Prentis PJ, Wilson JRU, Dormontt EE et al (2008) Adaptive evolution in invasive species. Trends Plant Sci 13:288–294
Reichard SH, Hamilton CW (1997) Predicting invasions of woody plants introduced into North America. Conserv Biol 11:193–203
Robert S, Ravigné V, Zapater M-F et al (2012) Contrasting introduction patterns among continents in the worldwide invasion of the banana fungal pathogen Mycosphaerella fijiensis. Mol Ecol 21:1098–1114
Roels SAB, Kelly JR (2011) Rapid evolution caused by pollinator loss in Mimulus guttatus. Evolution 65:2541–2552
Sakai AK, Allendorf FW, Holt JS et al (2001) The population biology of invasive species. Annu Rev Ecol Syst 32:305–332
Saleh D, Xu P, Shen Y et al (2012) Sex at the origin: an Asian population of the rice blast fungus Magnaporthe oryzae reproduces sexually. Mol Ecol 21:1330–1344
Sax DF, Stachowicz JJ, Brown JH et al (2007) Ecological and evolutionary insights from species invasions. Trends Ecol Evol 22:465–471
Singh RP, Hodson DP, Huerta-Espino J et al (2011) The emergence of Ug99 races of the stem rust fungus is a threat to world wheat production. Annu Rev Phytopathol 49:465–481
Stukenbrock EH, McDonald BA (2008) The origins of plant pathogens in agro-ecosystems. Annu Rev Phytopathol 46:75–100
Sutherland S (2004) What makes a weed a weed: life history traits of native and exotic plants in the USA. Oecologia 141:24–39
Travis JMJ, Hammershøj M, Stephenson C (2005) Adaptation and propagule pressure determine invasion dynamics: insights from a spatially explicit model for sexually reproducing species. Evol Ecol Res 7:37–51
Wiens JJ, Graham CH (2005) Niche conservatism: integrating evolution, ecology, and conservation biology. Annu Rev Ecol Evol Syst 36:519–539
Williamson MH, Fitter A (1996) The characters of successful invaders. Biol Conserv 78:163–170
Wloch DM, Szafraniec K, Borts RH, Korona R (2001) Direct estimate of the mutation rate and the distribution of fitness effects in the yeast Saccharomyces cerevisiae. Genetics 159:441–452
Zaykin DV, Pudovkin A, Weir BS (2008) Correlation-based inference for linkage disequilibrium with multiple alleles. Genetics 180:533–545
Acknowledgments
We are grateful to F. Halkett, BECPHY team of UMR BGPI, and people attending the various EMERFUNDIS workshops for helpful discussions. We also wish to thank S. Neuenschwander for providing quantiNEMO code and his help. The manuscript benefitted much from comments by the Editor, two reviewers and Mike Barfield. EB was funded by an ANR post-doctoral fellowship as part of the project EMERFUNDIS (ANR 07-BDIV-003) of the French “Agence Nationale de la Recherche” (ANR). This work was also supported by the French Agropolis Fondation (Labex Agro—Montpellier, BIOFIS Project Number 1001-001).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bazin, É., Mathé-Hubert, H., Facon, B. et al. The effect of mating system on invasiveness: some genetic load may be advantageous when invading new environments. Biol Invasions 16, 875–886 (2014). https://doi.org/10.1007/s10530-013-0544-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-013-0544-6