Abstract
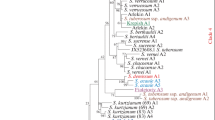
α-amylase is an important enzyme involved in starch degradation to provide energy to the germinating seedling. The present study was conducted to reveal structural and functional evolution of this gene among higher plants. Discounting polyploidy, most plant species showed only a single copy of the gene making multiple isoforms in different tissues and developmental stages. Genomic length of the gene ranged from 1472 bp in wheat to 2369 bp in soybean, and the size variation was mainly due to differences in the number and size of introns. In spite of this variation, the intron phase distribution and insertion sites were mostly conserved. The predicted protein size ranged from 414 amino acid (aa) in soybean to 449aa in Brachypodium. Overall, the protein sequence similarity among orthologs ranged from 56.4 to 97.4 %. Key motifs and domains along with their relative distances were conserved among plants although several species, genera, and class specific motifs were identified. The glycosyl hydrolase superfamily domain length varied from 342aa in soybean to 384aa in maize and sorghum while length of the C-terminal β-sheet domain was highly conserved with 61aa in all monocots and Arabidopsis but was 59aa in soybean and Medicago. Compared to rice, 3D structure of the proteins showed 89.8 to 91.3 % similarity among the monocots and 72.7 to 75.8 % among the dicots. Sequence and relative location of the five key aa required for the ligand binding were highly conserved in all species except rice.








Similar content being viewed by others
References
Baulcombe DC, Buffard D (1983) Gibberellic-acid-regulated expression of α-amylase and six other genes in wheat aleurone layers. Planta 157:493–501
Beck E, Ziegler P (1989) Biosynthesis and degradation of starch in higher plants. Ann Rev Plant Physiol 40:95–117
Biasini et al. (2014) SWISS-MODEL: modelling protein tertiary and quaternary structure using evolutionary information. Nucl Acids Res 42:(W1):W252-W258
Callis J, Ho TH (1983) Multiple molecular forms of the gibberellin-induced from the aleurone layers of barley seeds. Arch Biochem Biophys 1:224–234
Cheng CR, Oldach K, Mrva K, Mares D (2014) Analysis of high pI α-Amy-1 gene family members expressed in late maturity α-amylase in wheat (Triticum aestivum L). Mol Breed 33:519–529. doi:101007/s11032-01309968-z
Chrispeels MJ, Varner JE (1967) Gibberellic acid-enhanced synthesis and release of α-amylase and ribonuclease by isolated barley aleurone layers. Plant Physiol 42:398–406
Colovos C, Yeates TO (1993) Verification of protein structures: patterns of nonbonded atomic interactions. Protein Sci 2:1511–1519
Dhaliwal AK, Mohan A, Gill KS (2014) Comparative analysis of ABCB1 reveals novel structural and functional conservation between monocots and dicots. Front Plant Sci 5:1–10
Daussant J, Renard HA (1987) Development of different α-amylase isozymes, having high and low isoelectric points, during early stages of kernel development in wheat. J Cereal Sci 5:13–21
Emebiri LC, Oliver JR, Mrva K, Mares D (2010) Association mapping of late maturity α-amylase (LMA) activity in a collection of synthetic hexaploid wheat. Mol Breed 26:39–49
Farber GK, Petsko GA (1990) The evolution of α/β barrel enzymes. Trends Biochem Sci 15:228–234
Harvey BMR, Oaks A (1974) The hydrolysis of endosperm protein in Zea mays. Plant Physiol 53:453–457
Henrissat B (1991) A classification of glycosyl hydrolases based on amino acid sequence similarities. Biochem J 280:309–316
Higgins TJV, Jacobsen JV, Zwar JA (1982) Gibberellic acid and abscisic acid modulate protein synthesis and mRNA levels in barley aleurone layers. Plant Mol Biol 1:191–215
Janecek S (1992) New conserved amino acid region of α-amylases in the third loop of their (β/α) 8-barrel domains. Biochem J 288:1069–1070
Janecek S (1994) Parallel B/α-barrels of α-amylase, cyclodextrin glycosyltransferase and oligo-1,6-glucosidase versus the barrel of β-amylase: evolutionary distance is a reflection of unrelated sequences. FEBS Lett 353:119–123
Janecek S (2002) How many conserved sequence regions are there in the α-amylase family? Biologia 57:29–41
Janecek S (2009) Amylolytic enzymes-focus on the alpha-amylases from the Archaea and plants. Nova Biotechnolgia 9:5–25
Janecek S, Svenssen B, Henrissat B (1997) Domain evolution in the α-amylase family. J Mol Evol 45:322–331
Karrer EE, Litts JC, Rodriguez RL (1991) Differential expression of alpha amylase genes in germinating rice and barley seeds. Plant Mol Biol 16:797-805.
Kaur S, Dhugga KS, Gill K, Singh J (2016) Novel structural and functional motifs in cellulose synthase (CesA) genes of bread wheat (Triticum aestivum, L.). PLoS ONE 11(1):e0147046. doi:10.1371/journal.pone.0147046
Kelley LA, Mezulis S, Yates CM, Wass MN, Sternberg MJE (2015) The Phyre2 web portal for protein modelling, prediction and analysis. Nat Protoc 10:845–858
Kuriki T, Imanaka T (1999) The concept of the alpha-amylase family: structural similarity and common catalytic mechanism. J Biosci Bioeng 87:557–565
Lanahan MB, Ho TH, Rogers SW, Rogers JC (1992) A gibberellin response complex in cereal alpha-amylase gene promoters. Plant Cell 4:203–211
Larkin et al (2007) Clustal W and clustal X version 2.0. Bioinformatics 23:2947–2948
Luthy R, Bowie JU, Eisenberg D (1992) Assessment of protein models with three-dimensional profiles. Nature 356:83–85
MacGregor EA (1988) α-amylase structure and activity. J Prot Chem 7:399-415
MacGregor EA (1993) Relationship between structure and activity in α-amylase family of starch-metabolizing enzymes. Starch-Starke 45:232–237
MacGregor AE, Janecek S, Svensson B (2001) Relationship of sequence and structure to specifiity in the alpha-amylase family of enzymes. Biochim Biophys Acta 1546:1-20
Matsuura Y, Kusonoki M, Harada W, Kakaudo M (1984) Structure and possible catalytic residues of taka-amylase. J Biochem 95:697–702
Mundy J, Yamaguchi-Shinozaki K, Chua NH (1990) Nuclear proteins bind conserved elements in the abscisic acid-responsive promoter of a rice Rab gene. Proc Natl Acad Sci U S A 87:1406–1410
Murata T, Akazawa T, Fukuchi S (1968) Enzymic mechanism of starch breakdown in germinating rice seeds I; an analytical study. Plant Physiol 43:1899–1905
Nakajima R, Tadayuki I, Alba S (1986) Comparison of amino acid sequences of eleven different α-amylases. Appl Microbiol Biotech 23:355–360
Neill SD, Kumagai MH, Majumdar A, Huang N, Sutliff TD, Rodriguez RL (1990) The amylase genes in Oryza sativa: characterization of cDNA clones and mRNA expression during seed germination. Mol Genet Genomics 202:235–244
Rogozin IB, Sverdlov AV, Babenko VN, Koonin EV (2005) Analysis of evolution of exon-intron structure of eukaryotic genes. Brief Bioinform 6:118–134
Stamatakis A (2006) RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 22:2688–2690
Spratt BG, Pardee AB (1975) Penicillin-binding proteins and cell shape in E. coli. Nature 254:516–517
Staerz UD, Kanagawa O, Bevan MJ (1985) Hybrid antibodies can target sites for attack by T cells. Lett Nature 314:628–631
Svensson B (1994) Protein engineering in the alpha-amylase family: catalytic mechanism, substrate specificity, and stability. Plant Mol Biol 25:141–157
Swain RR, Dekker EE (1966) Seed germination studies ii. Pathways for starch degradation seedlings. Biochim Biophys Acta 122:87–100
Takata H, Kuriki T, Okada S, Takesada Y, Lizuka M, Minamiura N, Imanaka T (1992) Action of neopullulanase: neopullulanase catalyzes both hydrolysis and transglycosylation at alpha (1–4) and alpha (1–6)-glucosidic linkages. J Bio Chem 267:18447–18452
Tamura K, Stecher G, Peterson D, Filipski A, Kumar S (2013) MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol 30:2725–2729
Wass MN, Kelley LA, Sterberg MJE (2010) 3D LigandSite: predicting ligand binding site using similar structures. Nucl Acid Res 38:W469-W473
Ye Y, Godzik A (2003) Flexible structure alignment by chaining aligned fragment pairs allowing twists. Bioinformatics 19:11246–11255
Yomo H, Varner JE (1973) Control of the formation of amylases and proteases in the cotyledons of germinating peas. Plant Physiol 51:708–713
Acknowledgments
This work was supported by the USDA National Institute of Food and Agriculture, Hatch (project number WNP00449), and United States Agency for International Development Feed the Future Innovation Lab-Climate Resilient Wheat (Grant number AID-OAA-A-13-00008).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary materials
Below is the link to the electronic supplementary material.
Supplemental Table 1
Percent nucleotide similarity of OsAmy coding sequence (CDS) with ortholog in monocot and dicot species. (DOCX 15 kb)
Supplemental Table 2
Percent similarity of OsAmy with predicted protein in orthologs of monocot and dicot species along with their corresponding protein lengths. (DOCX 39 kb)
Supplemental Table 3
Length of GHS Domain and C-terminal β-sheet domain in monocot and dicot species. (DOCX 38 kb)
Supplemental Table 4
3D structure quality assessment parameters of α-amylase among monocots and dicots using SWISS-MODEL and SAVES server. (DOCX 58 kb)
Rights and permissions
About this article
Cite this article
Sethi, S., Saini, J.S., Mohan, A. et al. Comparative and evolutionary analysis of α-amylase gene across monocots and dicots. Funct Integr Genomics 16, 545–555 (2016). https://doi.org/10.1007/s10142-016-0505-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10142-016-0505-0