Abstract
Background
The standard of care for stage II/III gastric cancer in Japan is D2 dissection followed by adjuvant S-1 monotherapy. Outcome of patients with stage III disease remains unsatisfactory, calling for a more intensive adjuvant chemotherapy regimen, for which evidence in advanced/metastatic cancer research suggests S-1/cisplatin (CDDP) as a candidate. Although S-1/CDDP was poorly tolerated postoperatively in the previous trial, compliance was dramatically improved by insertion of one cycle of S-1 monotherapy, which delayed administration of CDDP by 6 weeks.
Methods
A feasibility study of post-gastrectomy S-1/CDDP was performed. Patients with stage III/IV gastric cancer were eligible. The first cycle of chemotherapy consisted of S-1 monotherapy, and intensive antiemetic drugs were prescribed when patients were administered CDDP. The primary endpoint was the completion rate of four cycles of S-1/CDDP. The secondary endpoints were the relative dose intensity, safety, progression-free survival time and overall survival time. Several criteria to skip, postpone or reduce the dose had been predetermined.
Results
Between 2010 and 2011, 33 patients were enrolled. Four patients had stage IIIA disease, 7 patients had stage IIIB disease, 11 patients had stage IIIC disease, and 11 patients had stage IV disease. The completion rate of the protocol treatment was 60.6 %. The relative dose intensity of S-1 was 77.3 % and that of CDDP was 72.3 %.
Conclusions
The protocol-specified delay in the administration of CDDP dramatically improved the relative drug intensity in the postoperative adjuvant setting, although the completion rate did not reach the expected level.
Similar content being viewed by others
Introduction
Although surgery remains the most important part of the multimodality strategy for gastric cancer, a randomized trial that explored extended lymphadenectomy has shown that a surgical procedure that is more extensive than D2 dissection does not improve the outcome in patients with gastric cancer even if surgical mortality is kept below 1 % [1]. Thus, making progress in adjuvant treatment is currently the only way to improve the outcome of resectable advanced gastric cancer. The Adjuvant Chemotherapy Trial of S-1 for Gastric Cancer (ACTS-GC trial) [2] showed that single-agent S-1 administered postoperatively for 1 year significantly improves the outcome of patients with stage II/III gastric cancer over treatment with surgery alone. In that trial, the effect of S-1 in preventing recurrence waned as the disease became more advanced, suggesting that some room for improvement in adjuvant therapy for the stage III category remains. Moreover, there are occasions when patients with stage IV cancer are treated by gastrectomy, and these patients are also in need of high-efficacy postoperative treatment.
Evidence in advanced gastric cancer indicates that S-1/CDDP is one of the candidates for this situation [3, 4]. Therefore, the authors previously conducted a feasibility study of postoperative chemotherapy with S-1/CDDP for patients with stage IV gastric cancer (CCOG 0703) [5]. Unfortunately, the trial was an utter failure since the relative dose intensity, the primary endpoint, was 37 % for S-1 and 40 % for CDDP, and the completion rate of 5 cycles of S-1/CDDP was 22.6 % (7/31). Since gastrectomy can have a detrimental effect on the appetite and oral food intake, postgastrectomy patients are particularly vulnerable to gastrointestinal tract toxicities such as nausea, vomiting and anorexia, which are often associated with the administration of CDDP [6, 7]. More recently, Takahari et al. [8] reported in a feasibility study that one 6-week cycle of S-1 monotherapy prior to the introduction of the S-1/CDDP regimen dramatically improved the completion rate of three courses of S-1/CDDP for post-gastrectomy patients with stage III disease. Furthermore, the long-term outcome of patients treated in that study was extremely promising with a 3-year overall survival of 84.5 % (95 % CI 72.3–91.6) for 63 patients with stage III cancer [9]. On the other hand, all institutions that participated in that study were established high-volume cancer centers where the treatment was delivered by medical oncologists. Since adjuvant chemotherapy for gastric cancer in Japan has more often been given by surgeons, it was intriguing to consider whether their success could be reproduced in the setting of community hospitals. In addition, approval of palonosetron and aprepitant, which were not available at the time of the study by Takahari et al., has enabled the introduction of more powerful combination antiemetic therapy to the clinic.
These facts prompted the authors to reevaluate the feasibility of postoperative S-1/CDDP in the community hospital setting, with the strategy of delaying administration of CDDP by replacing the first cycle with S-1 monotherapy and using state-of-the-art combination antiemetic therapy.
Patients and methods
Patient eligibility
Eligible patients had to meet all of the following criteria: (1) a confirmed diagnosis of gastric adenocarcinoma, (2) stage III or IV disease according to the Japanese Classification of Gastric Carcinoma, 14th edition (virtually equivalent to the Tumour Node Metastasis classification version 7), (3) age of 20–75 years, (4) gastrectomy performed within 8 weeks of initiation of chemotherapy, (5) no prior treatment besides surgery, (6) European Cooperative Oncology Group (ECOG) performance status of 0 to 1, (7) adequate organ functions, defined as white blood cell count 3,500–12,000/mm3, total neutrophil count 2,000/mm3 or more, platelet count 100,000/mm3 or more, hemoglobin 9.0 g/dl or more, total serum bilirubin <1.5 mg/dl, serum aspartate aminotransferase and alanine aminotransferase less than 100 IU/l, and creatinine clearance 60 ml/min or more. Patients had to have a life expectancy of more than 3 months, with no other active malignancies or uncontrolled concomitant diseases. Written informed consent was obtained from all participants after they had received a full explanation of the nature of the study.
The study was approved by the institutional review board of Nagoya University Hospital and all other hospitals belonging to the Chubu Clinical Oncology Group (CCOG) that participated in this multicenter trial.
Treatment plan and dose attenuation
At baseline, a complete medical history was taken, and a physical examination was performed. Laboratory assessment at baseline included blood cell counts, serum chemistry profiles, serum tumor markers (CEA, CA19-9) and urinalysis. Chemotherapy was to be started within 8 weeks after surgery. The first cycle of chemotherapy consisted of S-1 monotherapy. Patients received S-1 orally at the following doses twice daily for 4 weeks, followed by 2 weeks without chemotherapy. Patients with a body surface area of <1.25 m2 received 80 mg daily; those with a body surface area of 1.25 m2 to <1.5 m2 received 100 mg daily; those with a body surface area of 1.5 m2 or greater received 120 mg daily. After that, patients received S-1 for 3 weeks, followed by 2 weeks without chemotherapy. This 5-week cycle was repeated mainly in an outpatient setting. The exception was the intravenous administration of CDDP at 60 mg/m2 on day 8 of each cycle, for which the patients were to be admitted for 2–3 days and given continuous intravenous fluid administration. As for the antiemetics, palonosetron 0.75 mg and dexamethasone 12 mg were administered intravenously on day 8. In addition, oral aprepitant (125 mg on day 8 and 80 mg on days 9 and 10) and dexamethasone (8 mg, days 9–11) were prescribed. One cycle of S-1 and four cycles of S-1/CDDP were to be delivered as a protocol treatment, after which the patients with stage III disease were recommended to receive a further 6 months of chemotherapy with single-agent S-1. For patients with stage IV disease, there was no protocol-specified limitation to the number of cycles of S-1/CDDP to be given.
If the patients had hematological toxicity of grade 4 or greater, nonhematological toxicity of grade 3 or greater, or creatinine clearance of <60 ml/min before the start of a new course, the daily dose of S-1 was decreased from 120 to 100 mg, from 100 to 80 mg or from 80 to 50 mg, and the dose of CDDP was decreased by 10 mg/m2. If the patients failed to fulfill the criteria on day 1 of the new course, the new course was to be postponed until recovery. If such toxicity occurred on day 8, CDDP was to be skipped. Under these strict rules, the protocol treatment was to be discontinued in the event of (1) postponement of the new course for 3 weeks in a row, (2) dose reduction of S-1 or CDDP by two levels, (3) skipping CDDP for two cycles in a row, (4) other adverse events that were considered unmanageable, (5) withdrawal of consent from the patient or (6) disease recurrence or progression. Patients who failed the treatment were allowed to be given a second-line chemotherapy at the discretion of the surgeons/oncologists.
Adverse events were monitored by interviews, blood chemistry profiles and blood cell counts in every examination. All toxic effects were graded according to the National Cancer Institute Common Toxicity Criteria (NCI-CTC; version 4.0).
Endpoints and statistical analysis
The primary endpoint of this study was the completion rate of four cycles of S-1/CDDP; secondary endpoints were the relative drug intensity (percentage of the dose actually administered out of the planned dose calculated from the body surface area) during four cycles of treatment with S-1/CDDP, safety and overall survival time (OS).
According to the feasibility study by Takahari et al. [10], the completion rate of two courses and three courses of S-1/CDDP after one course of S-1 monotherapy was 95 and 81 %, respectively, for patients who underwent surgery for stage III cancer [10]. This suggests that a 10 % decline in the completion rate is expected by adding one course of S-1/CDDP. Since four courses of S-1/CDDP were planned after one course of S-1 monotherapy in the current study, the expected completion rate was determined to be 70 %. A minimum of 25 cases was needed to confine the 90 % confidence interval to ±15 %. Considering the possibilities of exclusion or dropout, the required sample size was raised to 30.
Results
Patient characteristics
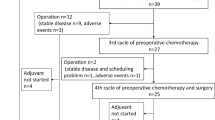
Thirty-three patients were enrolled between October 2011 and December 2012. The demographics and clinicopathological characteristics of the patients are shown in Table 1. The mean age of the patients was 65 years (range 40–75 years). The male:female ratio was 22:11. Eighteen patients underwent distal gastrectomy, and the other 15 received total gastrectomy. Four patients had stage IIIA disease, seven patients had stage IIIB disease, 11 patients had stage IIIC disease, and 11 patients had stage IV disease. Patients were followed for a median of 486 days or until death, and the 1-year survival rate was 82 %.
Compliance and relative drug intensity
The completion rate of one cycle of S-1 monotherapy and four cycles of S-1/CDDP was 60.6 % (20/33) among all patients enrolled. The completion rate of each cycle is shown in Table 2. The reasons for discontinuation of the treatment were adverse events in seven patients, patient refusal due to adverse events in two patients, disease progression in three patients and recurrence in one patient. Thus, the completion rate of the protocol treatment after excluding those who discontinued treatment because of recurrence or disease progression (n = 4) was 69.0 % (20/29).
The median relative dose intensity of S-1 was 77.3 % and that of CDDP was 72.3 %.
Treatment delay was needed in 18 patients (54.5 %). The most frequent reason for the delay was neutropenia. In addition, dose reduction was required in 12 patients (36.3 %) because of adverse events.
Toxicity
A total of 133 cycles from the 33 patients were assessable for toxicity (Table 3). The most frequent grade 3/4 hematological toxicity was neutropenia, observed in 30.3 % of the patients. Grade 3/4 anorexia was the most frequent nonhematological toxicity (30.3 %), followed by fatigue (18.2 %) and diarrhea (12.1 %). The frequency of grade 3/4 nausea was 9.1 % and that of grade 3/4 vomiting was 3.0 %.
Discussion
Since the current study was designed primarily to look at the feasibility of S-1/CDDP in the postoperative phase, patients with both stage III and IV disease were eligible. One could argue that patients with stage IV disease suffer from a more advanced disease and greater cancer burden, which may influence the tolerability to chemotherapy. However, stage IV cancer in the current Japanese practice usually undergoes surgery only under the condition that residual disease could be kept to the minimum. Thus, we consider that comparisons in terms of feasibility could be made between the current results and those of CCOG0703 [5], a trial exclusively of resected stage IV cancer, and the study by Takahari et al. [8], which only looked at stage III cancer. All 8 patients with gross metastases (3 hepatic and 5 peritoneal) in the current study underwent metastatectomy along with gastrectomy; consequently, only 6 patients received R1 resection (positive cytology results of the peritoneal washes in 4 and microscopically positive resection margins in 3), while none received R2 resection. Thus, the target of chemotherapy in both stage III and stage IV disease was microscopic residual disease.
However, disease progression in the absence of critical adverse events occurred more frequently among stage IV patients, and these did interfere with the evaluation of tolerability in the current study. The completion rate of four cycles of S-1/CDDP was 60.6 % (20/33); the expected completion rate of 70 % was not achieved at least in part because of the four patients who stopped treatment because of disease recurrence. Nevertheless, the completion rate was markedly higher than in the previous CCOG0703 trial by the same study group at 22.6 % [5]. The relative drug intensity of S-1 and that of CDDP were also markedly higher in this study (S-1 77.3 %, CDDP 72.3 %) than in the previous trial (S-1 37 %, CDDP 40 %).
Although overall survival was one of the secondary endpoints, the authors decided to publish the current data before waiting for the availability of mature data because (1) the survival data of the mixed population of stage III/IV patients render comparison with previous studies inadequate, (2) the promising outcome by the same treatment for stage III cancer has already been reported from a study involving a larger population [9], and data on feasibility are required immediately to design the next generation of trials testing the perioperative therapy for stage III cancer.
The combination antiemetic treatment of palonosetron, aprepitant and dexamethasone was recommended for patients receiving high emetic risk intravenous chemotherapy including CDDP, especially, in patients treated with CDDP ≥ 50 mg/m2 [10]. Patients who were entered into the current study were expected to benefit from the availability of these novel antiemetics, which had not been approved during the previous studies [5, 8]. However, the incidence of grade 3/4 nausea, vomiting, anorexia and general fatigue was observed in 9.1, 3, 30.3 and 18.2 % in the current study as opposed to 10, 0 and 23 and 10 % in CCOG 0703 [5]. In other words, the incidence of severe gastrointestinal toxicities per se was not markedly reduced. On the other hand, six patients in the CCOG 0703 discontinued treatment due to patient refusal because of the adverse events, which did not reach levels rendering the patients ineligible for further treatment, whereas this happened only in two patients in the current study. In the previous study, seven patients had to discontinue the protocol treatment because of a delay of >3 weeks to starting a new cycle due to poor recovery from adverse events, but this phenomenon was not observed in the current study. These findings suggest that the patients suffered from a similar extent of emesis but were able to tolerate this owing to enhanced recovery from the surgical intervention through the additional 6 weeks provided in the current study before the first administration of CDDP.
There is a definite weakness in the S-1/CDDP combination, in addition to the adverse events, that admission for hydration and management of emesis is usually desired for patients with advanced cancer and is mandatory for those who receive the treatment postoperatively. This weakness becomes more critical when a large number of cycles are needed. There is no consensus regarding the optimal number of cycles to be given in the adjuvant setting. Since the treatment continued for 6 months with capecitabine and oxaliplatin in the successful CLASSIC trial for gastric cancer [11], and 6 months of adjuvant chemotherapy is quite usual in several other cancer types, one cycle of S-1 and four cycles of S-1/CDDP, which amounts to 6 months of treatment, could be considered sufficient. However, since adjuvant chemotherapy with S-1 monotherapy for 12 months is the current standard of care in Japan [2], patients with stage III disease were recommended to receive a further 6 months of chemotherapy with single-agent S-1 at this time. The excellent outcome reported from the study by Takahari et al. [9] suggests that the treatment by S-1/CDDP could be reduced to three cycles. Determining the length of treatment and subsequent randomized comparison with S-1 monotherapy to show significant superiority are needed to confirm that this promising combination should be recommended despite the weaknesses.
Finally, evidence suggests that oxaliplatin is at least not inferior to cisplatin as a platinum agent against gastric cancer [12]. A combination of oxaliplatin and S-1 could be a promising option [13] along with oxaliplatin and capecitabine [11], for which phase III evidence already exists. However, comparison of the pattern of disease failure in the ACTS-GC trial and the CLASSIC trial suggests that S-1 is effective in eliminating cancer cells in the peritoneal cavity, which may cause peritoneal dissemination, whereas the oxaliplatin and capecitabine combination effectively prevented hematogenous metastasis. Moreover, in an attempt to classify gastric cancer based on the gene expression pattern, Tan et al. [14] found that the two subtypes of gastric cancer respond differently to platinum agents, with a tendency for higher sensitivity to cisplatin being observed in diffuse type cancer. These findings suggest that it is still meaningful to explore cisplatin in the postoperative adjuvant setting alongside oxaliplatin.
To conclude, in the current feasibility study involving patients with stage III/IV gastric cancer, postoperative treatment consisting of one cycle of S-1 monotherapy followed by 4 cycles of S-1/CDDP was tolerated by 60.6 % of all 33 patients who were entered onto the trial and by 69 % if the 4 patients who stopped treatment because of cancer recurrence were excluded. Our results support the strategy of delaying the introduction of CDDP in the postoperative adjuvant setting, and under that strategy, postoperative adjuvant S-1/CDDP remains a candidate to be explored for stage III gastric cancer.
References
Sasako M, Sano T, Yamamoto S, Kurokawa Y, Nashimoto A, Kurita A, et al. D2 lymphadenectomy alone or with para-aortic nodal dissection for gastric cancer. N Engl J Med. 2008;359:453–62.
Sasako M, Sakuramoto S, Katai H, Kinoshita T, Furukawa H, Yamaguchi T, et al. Five-year outcomes of a randomized phase III trial comparing adjuvant chemotherapy with S-1 versus surgery alone in stage II or III gastric cancer. J Clin Oncol. 2011;29:4387–93.
Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2010 (ver. 3). Gastric Cancer. 2011;14:113–23.
Koizumi W, Narahara H, Hara T, Takagabe A, Akiya T, Takagi M, et al. S-1 plus cisplatin versus S-1 alone for first-line treatment of advanced gastric cancer (SPIRITS trial): a phase III trial. Lancet Oncol. 2008;9:215–21.
Kodera Y, Ishiyama A, Yoshikawa T, Kinoshita T, Ito S, Yokoyama H, et al. A feasibility study of postoperative chemotherapy with S-1 and cisplatin (CDDP) for gastric carcinoma (CCOG0703). Gastric Cancer. 2010;13:197–203.
Gralla RJ, Osoba D, Kris MG, Kirkbride P, Hesketh PJ, Chinnery LW, et al. Recommendations for the use of antiemetics: evidence-based, clinical practice guidelines. American Society of Clinical Oncology. J Clin Oncol. 1999;17:2971–94.
Roila F, Herrstedt J, Aapro M, GRalla RJ, Einhorn LH, Ballatori E, et al. Guideline update for MASCC and ESMO in the prevention of chemotherapy- and radiotherapy-induced nausea and vomiting: results of the Perugia consensus conference. Ann Oncol. 2010;Suppl 5:232–43.
Takahari D, Hamaguchi T, Yoshimura K, Kinoshita T, Ito S, Yokoyama H, et al. Feasibility study of adjuvant chemotherapy with S-1 plus cisplatin for gastric cancer. Cancer Chemother Pharmacol. 2011;67:1423–8.
Takahari D, Hamaguchi T, Yoshimura K, Katai H, Ito S, Fuse N, et al. Survival analysis of adjuvant chemotherapy with S-1 plus cisplatin for stage III gastric cancer. Gastric Cancer. 2014;14:383–6.
National Comprehensive Cancer Network: NCCN clinical practice guidelines in oncology: antiemesis. ver. 3. 2011.
Bang YJ, Kim Y-W, Yang H-K, Chung HC, Park Y-K, Lee KH, et al. Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): a phase 3 open-label, randomized controlled trial. Lancet. 2012;379:315–21.
Cunningham D, Starling N, Rao S, Iveson T, Nicolson M, Coxon F, et al. Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med. 2008;358:36–46.
Koizumi W, Takiuchi H, Yamada Y, Boku N, Fuse N, Muro K, et al. Phase II study of oxaliplatin plus S-1 as first-line treatment for advanced gastric cancer (G-SOX study). Ann Oncol. 2010;21:1001–5.
Tan IB, Ivanova T, Lim KH, Ong CW, Deng N, Lee J, et al. Intrinsic subtypes of gastric cancer, based on gene expression pattern, predict survival and respond differently to chemotherapy. Gastroenterology. 2011;141:476–85.
Author information
Authors and Affiliations
Corresponding author
Additional information
For the Chubu Clinical Oncology Group.
Rights and permissions
About this article
Cite this article
Kurimoto, K., Ishigure, K., Mochizuki, Y. et al. A feasibility study of postoperative chemotherapy with S-1 and cisplatin (CDDP) for stage III/IV gastric cancer (CCOG 1106). Gastric Cancer 18, 354–359 (2015). https://doi.org/10.1007/s10120-014-0384-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10120-014-0384-9