Abstract
Tumor necrosis factor α (TNF-α) inhibitors ranked highest in German pharmaceutical expenditure in 2011. Their most important application is the treatment of rheumatoid arthritis (RA). Our objective is to analyze cost per responder of TNF-α inhibitors for RA from the German Statutory Health Insurance funds' perspective. We aim to conduct the analysis based on randomized comparative effectiveness studies of the relevant treatments for the German setting. For inclusion of effectiveness studies, we require results in terms of response rates as defined by European League Against Rheumatism (EULAR) or American College of Rheumatology (ACR) criteria. We identify conventional triple therapy as the relevant comparator. We calculate cost per responder based on German direct medical costs. Direct clinical comparisons could be identified for both etanercept and infliximab compared to triple therapy. For infliximab, cost per responder was 216,392 euros for ACR50 and 432,784 euros for ACR70 responses. For etanercept, cost per ACR70 responder was 321,527 euros. Cost was lower for response defined by EULAR criteria, but data was only available for infliximab. Cost per responder is overestimated by 40 % due to inclusion of taxes and mandatory rebates in German drugs' list prices. Our analysis shows specific requirements for cost-effectiveness analysis in Germany. Cost per responder for TNF-α treatment in the German setting is more than double the cost estimated in a similar analysis for the USA, which measured against placebo. The difference in results shows the critical role of the correct comparator for a specific setting.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rheumatoid arthritis (RA) is the most common chronic inflammatory systemic disease. Its socioeconomic relevance is characterized by a high prevalence in industrialized countries combined with the productivity loss associated with the disease.
The goal of the therapy is early disease control and induction of sustained remission [1]. Patients can be treated with conventional or biological disease-modifying therapies. Methotrexate (MTX) is the most commonly used disease-modifying antirheumatic drug (DMARD). Tumor necrosis factor α (TNF-α) inhibitors are the most popular biological agents approved for the treatment of RA after failure of conventional DMARDs. Biological agents incur higher direct treatment costs.
Germany is the most populous country in the European Union and its most important market for biological agents. The main payer in the German health care system is the Statutory Health Insurance (SHI). The SHI funds provide insurance to 90 % of the German population. The SHI funds have more than 70 million members. With more than EUR 1 billion expenditure, TNF-α inhibitors ranked highest in the SHI funds' pharmaceutical expenditure in 2011 [2].
For the German population, overall prevalence of inflammatory arthritis is estimated at 3.4 % [3]. The prevalence of RA is 1 %, i.e., approximately 800,000 people [4]. The percentage of patients treated with TNF-α inhibitors has been rising from 2 % in 2000 to 20 % in 2008 [5]. In 2011, adalimumab, etanercept, and infliximab were the most popular biological agents by sales.
High prevalence and high pharmaceutical spending motivate a cost-effectiveness analysis (CEA) of TNF-α therapies for the German setting. We aim to conduct a CEA considering the special requirements for health economic analysis in Germany.
Methods
We choose cost per responder as the most suitable measure of cost-effectiveness for the German setting. Quality-adjusted life years are not used in the German context due to legal concerns [6]. Cost-effectiveness in terms of cost per function score unit is problematic as the commonly used Health Assessment Questionnaire is not cardinally scaled.
We conduct our analysis from the SHI funds' point of view. Reporting from the main payer's perspective has been suggested by the Institute for Quality and Efficiency in Health Care for CEA in Germany.
For inclusion of effectiveness studies, we require results in terms of response rates as defined either by European League Against Rheumatism (EULAR) criteria (EULAR good response, EULAR moderate to good response) or by American College of Rheumatology (ACR) criteria (ACR50 response, ACR70 response).
First-line biological therapy is not reimbursed by the German SHI funds unless individual reasons imply poor response or toxicity of DMARDs. German guidelines require treatment for at least 6 months and failure of at least two DMARDs including MTX before the initiation of biological therapy [7].
While MTX monotherapy is chosen as the comparator in most CEAs of biological treatments, this would not reflect German clinical practice. By definition, patients who are eligible for MTX monotherapy would not be treated with TNF-α inhibitors in the German setting. Only MTX refractory patients would be treated with biological therapies. The German Federal Joint Committee's (Gemeinsamer Bundesausschuss (GBA)) requirements include identification of the correct comparator for the specific patient population [8].
If MTX monotherapy is not effective, DMARD combination therapy can be initiated as an alternative to biological treatments [9]. DMARD combination therapy has been shown to be more effective than MTX monotherapy [10]. We identify conventional triple therapy as the most clinically relevant alternative to biological therapy as shown by 2011 SHI prescription data [2]. The combination of MTX, sulfasalazine, and hydroxychloroquine has been shown to be more effective than MTX monotherapy by O'Dell [11]. It is the most suitable comparator to reflect a treatment alternative to biological therapy in German clinical practice.
Therefore, we search PubMed for clinical comparisons of TNF-α therapy and triple therapy. We conduct our analysis based on direct clinical comparisons. Direct clinical comparisons offer a higher level of evidence than indirect comparisons according to GBA's requirements [8].
All clinical trials are included if they feature at least one study arm with biological therapy and one arm with triple therapy. Initial treatment needs to be MTX monotherapy or failure of MTX monotherapy has to be an inclusion criterion for patients.
We search PubMed for the search terms “adalimumab, sulfasalazine, hydroxychloroquine, and rheumatoid arthritis.” We screen all hits as well as their references for further studies. The search produced 17 English language results. One clinical trial could be identified which compared adalimumab to triple therapy [12]. Application of the same search terms to infliximab and etanercept produced 40 and 34 hits. Screening of the hits resulted in the identification of three clinical trials for infliximab [13–17] and two clinical trials for etanercept [18, 19].
We calculate cost per responder based on German direct medical costs for the treatments applied in the trials. Cost calculations include 2013 drug costs and treatment costs (administration and monitoring cost and screening cost before initiation of the therapy) according to German SHI payment conditions.
We report the sensitivity to the national specifics of German drug pricing. Contrary to other countries, Germany applies the full 19 % value-added tax (VAT) to all pharmaceuticals. We provide results without VAT for better international comparability as shown in Table 1.
In the last decade, the German legislator has routinely applied mandatory rebates to nonreference price group pharmaceuticals. The SHI funds' savings due to mandatory rebates are not reflected by manufacturers' list prices. The manufacturers currently have to reimburse the SHI funds for 16 % of list prices. We report results reflecting the 16 % rebate as shown in Table 1.
Results
Four out of six identified trials were excluded because they compared combinations of biological and triple therapy rather than comparing biological to triple therapy. The OPERA study compares adalimumab and MTX combination therapy plus possible step-up O’Dell triple therapy to MTX (and adalimumab placebo) therapy plus possible step-up O’Dell triple therapy [12]. While the placebo arm reflects German clinical practice, the adalimumab arm includes both biological and triple conventional therapy. The NEO-RACo study added infliximab on top of an existing triple therapy strategy [15]. O’Dell analyzed the addition of etanercept to either component of conventional triple therapy [18]. The BeSt study includes four actively managed study arms (DMARD sequential monotherapy, DMARD step-up combination therapy, DMARD initial combination therapy, initial infliximab, and MTX combination therapy) [13, 14]. While providing a valuable comparison of four different treatment approaches, the active study design ultimately leads to infliximab combination therapy in all study arms. Each study arm has an individual treatment plan that differs both in medication and timing. The study designs are not suitable to compare biological therapy to conventional therapy for the purpose of our analysis.
The Swefot trial compares O'Dell's triple therapy to infliximab and MTX combination therapy after failure of MTX monotherapy [16, 17]. The Swefot trial reflects two important characteristics of German practice: failure of MTX therapy (even though failure of a second DMARD would be required in Germany) and comparison to O'Dell's triple treatment.
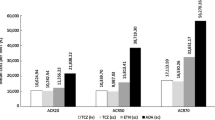
The Swefot trial reports response rates according to both ACR criteria and to EULAR criteria. Cost of the study medication in the German setting is shown in Table 1.
Given the 12 months results of the Swefot trial, the incremental cost-effectiveness ratios (ICER) for ACR response under infliximab combination therapy compared to O'Dell's triple therapy are 216,392 euros (ACR50) and 432,784 euros (ACR70). For EULAR criteria, the ICERs are 154,566 euros for a good response and 196,720 euros for a good to moderate response (Table 2).
The Treatment of Early Aggressive Rheumatoid Arthritis (TEAR) trial compares O'Dell's triple therapy to etanercept and MTX combination therapy, both as initial therapies and as step-up therapies after the failure of initial MTX monotherapy after 6 months [19]. Like the Swefot trial, the two step-up arms reflect the failure of one DMARD. The trial reports response according to ACR criteria only. After 2 years, only ACR70 response significantly differed between both study arms. The ICER for a ACR70 response is 321,527 euros (projected for 1 year) (Table 3).
Discussion
A similar analysis for the USA estimated cost per responder for infliximab at $92,081 by ACR50 criteria and at $152,471 by ACR70 criteria and for etanercept at $63,137 (ACR50) and at $135,085 (ACR70) against placebo [20]. The costs estimated in our study are more than twice as high. This difference can mainly be attributed to the use of triple therapy rather than placebo as a comparator for the German setting. Our results show that cost per responder would be underestimated if the relevant comparator was not taken into account.
However, all German results are overestimated by 40 % when including VAT and mandatory rebates in the cost calculations. This needs to be taken into account when reporting results based on German list prices.
If drug costs are adapted and the specific relevant comparator is taken into account, our results can be transferred to many other countries, which do not use quality-adjusted life years as part of policy decisions due to legal or political concerns.
For the Swefot trial, our calculations are based on 12-month data. However, both trials show little evidence for any significant clinical advantage of TNF-α therapy after 2 years. Under the conditions of these trials, TNF-α therapy would not be cost-effective for MTX refractory patients.
Current EULAR recommendations do not explicitly recommend conventional DMARD combination therapy [21]. A more prominent role of conventional combination therapy could contribute to significant direct medical cost savings.
At 5 years, the BeSt study has shown that 19 % of patients are in drug-free remission after initial treatment with infliximab [22]. Comparable rates might be achieved even if not applied as first-line therapy. The possibility for drug-free remission could have an important impact on the results of CEA.
Accounting for prevented disability in long-term analysis can substantially change the results of CEA if conducted from a societal perspective. Initial biological therapy with adalimumab showed an advantage in radiographic progression over MTX monotherapy even if adalimumab therapy was discontinued after 1 year [23].
Limitations
If no response could be achieved, the Swefot trial allowed switching the therapy from infliximab to etanercept and from triple therapy to cyclosporin A. These adjustments are not reflected by our cost calculations. However, the variations in costs do not alter our conclusions. The huge cost difference between biological and conventional therapy does not significantly change for either switch.
The data discovered to directly base CEA upon is very limited. The Swefot trial only includes 258 patients over the course of 2 years. The setup of the trial does not fully reflect German guidelines. The same critique applies to CEA based on the TEAR trial with 379 patients in the step-up arms.
Conclusions
Our analysis has shown specific requirements for CEA for the German market. CEA by cost per responder is a viable option for jurisdictions, which do not use quality-adjusted life years. Our results reflect GBA's requirements for relevant comparators as currently practiced in its early benefit assessments for new approvals in Germany [8].
While our analysis focused on the agents with the highest budget impact in 2011, GBA selected tocilizumab, golimumab, and certolizumab pegol for the first benefit assessment of biological agents for RA in 2014 [24]. These agents are in an earlier stage of their product lifecycle. Due to their expected future budget impact, their manufacturers are required to negotiate a rebate with the SHI funds based on GBA's benefit assessment. Our analysis has shown that the choice of the comparator will play a critical role.
Cost per responder for the German setting is more than double the cost estimated in a similar analysis for the USA, which measured against placebo. However, German results are overestimated by 40 % when calculating based on the drugs' list prices.
Conclusions need to be drawn with caution. Recent research has shown the possibilities of long-term drug-free remission and improved radiographic progression with early biological treatment. Further research is required to determine the long-term cost-effectiveness of biological approaches to treatment of RA.
References
Scott DL, Wolfe F, Huizinga TWJ (2010) Rheumatoid arthritis. Lancet 376(9746):1094–1108. doi:10.1016/S0140-6736(10)60826-4
Schwabe U, Paffrath D (2012) Arzneiverordnungs-Report 2012. Springer, Berlin
Schneider S, Schmitt G, Richter W (2006) Prevalence and correlates of inflammatory arthritis in Germany: data from the First National Health Survey. Rheumatol Int 27(1):29–38
Wasmus A, Kindel P, Mattussek S, Raspe HH (1989) Activity and severity of rheumatoid arthritis in Hannover/FRG and in one regional referral center. Scand J Rheumatol Suppl 79:33–44
Zink A, Huscher D, Schneider M (2010) How closely does rheumatology treatment follow the guidelines?: ambition and reality. Z Rheumatol 69(4):318–326. doi:10.1007/s00393-009-0522-7
Caro J, Nord E, Siebert U et al (2010) The efficiency frontier approach to economic evaluation of health-care interventions. Health Econ 19(10):1117–1127
Krüger K, Wollenhaupt J, Albrecht K et al (2012) German 2012 guidelines for the sequential medical treatment of rheumatoid arthritis : adapted EULAR recommendations and updated treatment algorithm. Z Rheumatol 71(7):592–603. doi:10.1007/s00393-012-1038-0
Gemeinsamer Bundesausschuss (2012) [Rules of Procedure]. BAnz AT 06.09.2012 B5
Krüger K (2011) Combination therapy using methotrexate with DMARDs or biologics—current status. Z Rheumatol 70(2):114–122. doi:10.1007/s00393-010-0684-3
Choy EHS, Smith C, Doré CJ, Scott DL (2005) A meta-analysis of the efficacy and toxicity of combining disease-modifying anti-rheumatic drugs in rheumatoid arthritis based on patient withdrawal. Rheumatology 44(11):1414–1421. doi:10.1093/rheumatology/kei031
O'Dell JR, Haire CE, Erikson N et al (1996) Treatment of rheumatoid arthritis with methotrexate alone, sulfasalazine and hydroxychloroquine, or a combination of all three medications. New Engl J Med 334(20):1287–1291. doi:10.1056/NEJM199605163342002
Hørslev-Petersen K, Hetland ML, Junker P et al (2013) Adalimumab added to a treat-to-target strategy with methotrexate and intra-articular triamcinolone in early rheumatoid arthritis increased remission rates, function and quality of life. The OPERA Study: an investigator-initiated, randomised, double-blind, parallel-group, placebo-controlled trial. Ann Rheum Dis 0:1–8. doi:10.1136/annrheumdis-2012-202735
Goekoop-Ruiterman YPM, de Vries-Bouwstra JK, Allaart CF et al (2008) Clinical and radiographic outcomes of four different treatment strategies in patients with early rheumatoid arthritis (the BeSt study): a randomized, controlled trial. Arthritis Rheum-US 58(2 Suppl):S126–S135. doi:10.1002/art.23364
Goekoop-Ruiterman YPM, de Vries-Bouwstra JK, Allaart CF et al (2005) Clinical and radiographic outcomes of four different treatment strategies in patients with early rheumatoid arthritis (the BeSt study): a randomized, controlled trial. Arthritis Rheum-US 52(11):3381–3390. doi:10.1002/art.21405
Leirisalo-Repo M, Kautianinen H, Laasonen L et al (2012) Infliximab for 6 months added on combination therapy in early rheumatoid arthritis: 2-year results from an investigator-initiated, randomised, double-blind, placebo-controlled study (the NEO-RACo Study. Ann Rheum Dis 0:1–7. doi:10.1136/annrheumdis-2012-201365
van Vollenhoven RF, Ernestam S, Geborek P et al (2009) Addition of infliximab compared with addition of sulfasalazine and hydroxychloroquine to methotrexate in patients with early rheumatoid arthritis (Swefot trial): 1-year results of a randomised trial. Lancet 374(9688):459–466. doi:10.1016/S0140-6736(09)60944-2
van Vollenhoven RF, Geborek P, Forslind K et al (2012) Conventional combination treatment versus biological treatment in methotrexate-refractory early rheumatoid arthritis: 2 year follow-up of the randomised, non-blinded, parallel-group Swefot trial. Lancet 379(9827):1712–1720. doi:10.1016/S0140-6736(12)60027-0
O'Dell JR, Petersen K, Leff R et al (2006) Etanercept in combination with sulfasalazine, hydroxychloroquine, or gold in the treatment of rheumatoid arthritis. J Rheumatol 33(2):213–218
Moreland LW, O'Dell JR, Paulus HE et al (2012) A randomized comparative effectiveness study of oral triple therapy versus etanercept plus methotrexate in early, aggressive rheumatoid arthritis. Arthritis Rheum-US. doi:10.1002/art.34498
Liu Y, Wu EQ, Bensimon AG et al (2012) Cost per responder associated with biologic therapies for Crohn's disease, psoriasis, and rheumatoid arthritis. Adv Ther 29(7):620–634. doi:10.1007/s12325-012-0035-7
Smolen JS, Landewé R, Breedveld FC et al (2010) EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs. Ann Rheum Dis 69:964–975. doi:10.1136/ard.2009.126532
Klarenbeek NB, Güler-Yüksel M, van der Kooij SM et al (2011) The impact of four dynamic, goal-steered treatment strategies on the 5-year outcomes of rheumatoid arthritis patients in the BeSt study. Ann Rheum Dis 70(6):1039–1046. doi:10.1136/ard.2010.141234
Detert J, Bastian H, Listing J et al (2012) Induction therapy with adalimumab plus methotrexate for 24 weeks followed by methotrexate monotherapy up to week 48 versus methotrexate therapy alone for DMARD-naive patients with early rheumatoid arthritis: HIT HARD, an investigator-initiated study. Ann Rheum Dis. doi:10.1136/annrheumdis-2012-201612
Hecken J (2013) [Decision of the Federal Joint Committee on the initiation of benefit assessments for existing pharmaceuticals according to § 35a par. 6 SGB V and Chapter 5 § 16 VerfO]. BAnz AT 07.05.2013 B4
Disclosures
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Gissel, C., Repp, H. Cost per responder of TNF-α therapies in Germany. Clin Rheumatol 32, 1805–1809 (2013). https://doi.org/10.1007/s10067-013-2332-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-013-2332-1