Abstract
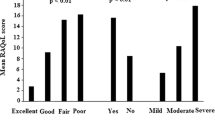
The Rheumatoid Arthritis Quality of Life (RAQoL) questionnaire is the first needs-based instrument specifically designed to measure quality of life (QoL) of patients with rheumatoid arthritis (RA). The aims of our study were to develop an Argentinean version of the RAQoL and to determine its reproducibility, validity, and sensitivity to change in patients with RA. Translation process was performed according to internationally accepted methodology. Internal consistency and test–retest reliability were calculated. Criterion and construct validity were assessed by comparing the RAQoL with parameters of disease activity, the Health Assessment Questionnaire (HAQ), and the Medical Outcomes Study 36-item health survey (SF-36) questionnaire. Sensitivity to change was measured at 6–12 months using standardized response mean (SRM). The minimal important change was defined as a change of 1 or 1.96 times the standard error of measurement. A total of 97 patients with RA were included. Cronbach’s α was 0.93, and test–retest reliability was 0.95. The RAQoL showed moderate to strong correlation with parameters of disease activity, the HAQ, and the SF-36. Functional status was the main determinant of patients’ level of QoL. The SRM of the RAQoL was 0.24. Agreement between 20 % improvement in RAQoL and ACR20 response was moderate. Minimal important change was 2.2 (1 SEM) or 4.3 (1.96 SEM). The Argentinean version of the RAQoL is the first Spanish translation of this questionnaire. Our findings show it to be valid, reliable, and sensitive to changes in RA clinical status.


Similar content being viewed by others
References
Kaplan RM (2007) The future of outcomes measurement in rheumatology. Am J Manag Care 13(Suppl 9):S252–S255
Bergner M, Bobbitt RA, Pollard WE, Martin DP, Gilson BS (1976) The sickness impact profile: validation of a health status measure. Med Care 14(1):57–67
Augustovski FA, Irazola VE, Velazquez AP, Gibbons L, Craig BM (2009) Argentine valuation of the EQ-5D health states. Value Health 12(4):587–596
Houssien DA, McKenna SP, Scott DL (1997) The Nottingham Health Profile as a measure of disease activity and outcome in rheumatoid arthritis. Br J Rheumatol 36(1):69–73
Augustovski FA, Lewin G, Elorrio EG, Rubinstein A (2008) The Argentine–Spanish SF-36 Health Survey was successfully validated for local outcome research. J Clin Epidemiol 61(12):1279–1284
Pincus T, Wolfe F (2000) An infrastructure of patient questionnaires at each rheumatology visit: improving efficiency and documenting care. J Rheumatol 27(12):2727–2730
Cooper JK, Kohlmann T, Michael JA, Haffer SC, Stevic M (2001) Health outcomes. New quality measure for Medicare. Int J Qual Health Care 13(1):9–16
Wells G, Boers M, Shea B, Tugwell P, Westhovens R, Saurez-Almazor M et al (1999) Sensitivity to change of generic quality of life instruments in patients with rheumatoid arthritis: preliminary findings in the generic health OMERACT study. OMERACT/ILAR Task Force on Generic Quality of Life. Life Outcome Measures in Rheumatology. International League of Associations for Rheumatology. J Rheumatol 26(1):217–221
Whalley D, McKenna SP, de Jong Z, van der Heijde D (1997) Quality of life in rheumatoid arthritis. Br J Rheumatol 36(8):884–888
de Jong Z, van der Heijde D, McKenna SP, Whalley D (1997) The reliability and construct validity of the RAQoL: a rheumatoid arthritis-specific quality of life instrument. Br J Rheumatol 36(8):878–883
McKenna SP, Hunt SM (1992) A new measure of quality of life in depression: testing the reliability and construct validity of the QLDS. Health Policy 22(3):321–330
Cox SR, McWilliams L, Massy-Westropp N, Meads DM, McKenna SP, Proudman S (2007) Adaptation of the RAQoL for use in Australia. Rheumatol Int 27(7):661–666
Hedin PJ, McKenna SP, Meads DM (2006) The Rheumatoid Arthritis Quality of Life (RAQoL) for Sweden: adaptation and validation. Scand J Rheumatol 35(2):117–123
Bejia I, Salem KB, Touzi M, Bergaoui N. Validation of the Tunisian version of the rheumatoid arthritis quality of life (RAQoL) questionnaire. Clin Rheumatol. 2006.
Tammaru M, McKenna SP, Meads DM, Maimets K, Hansen E (2006) Adaptation of the rheumatoid arthritis quality of life scale for Estonia. Rheumatol Int 26(7):655–662
Tammaru M, Strompl J, Maimets K, Hanson E (2004) The value of the qualitative method for adaptation of a disease-specific quality of life assessment instrument: the case of the Rheumatoid Arthritis Quality of Life Scale (RAQoL) in Estonia. Health Qual Life Outcomes 2:69
Kutlay S, Kucukdeveci AA, Gonul D, Tennant A (2003) Adaptation and validation of the Turkish version of the Rheumatoid Arthritis Quality of Life Scale. Rheumatol Int 23(1):21–26
Eberhardt K, Duckberg S, Larsson BM, Johnson PM, Nived K (2002) Measuring health related quality of life in patients with rheumatoid arthritis—reliability, validity, and responsiveness of a Swedish version of RAQoL. Scand J Rheumatol 31(1):6–12
Tijhuis GJ, de Jong Z, Zwinderman AH, Zuijderduin WM, Jansen LM, Hazes JM et al (2001) The validity of the Rheumatoid Arthritis Quality of Life (RAQoL) questionnaire. Rheumatology (Oxford) 40(10):1112–1119
Neville C, Whalley D, McKenna S, Le Comte M, Fortin PR (2001) Adaptation and validation of the rheumatoid arthritis quality of life scale for use in Canada. J Rheumatol 28(7):1505–1510
Thorsen H, Hansen TM, McKenna SP, Sorensen SF, Whalley D (2001) Adaptation into Danish of the Stanford Health Assessment Questionnaire (HAQ) and the Rheumatoid Arthritis Quality of Life Scale (RAQoL). Scand J Rheumatol 30(2):103–109
Linde L, Sorensen J, Ostergaard M, Horslev-Petersen K, Hetland ML (2008) Health-related quality of life: validity, reliability, and responsiveness of SF-36, 15D, EQ-5D [corrected] RAQoL, and HAQ in patients with rheumatoid arthritis. J Rheumatol 35(8):1528–1537
Whalley D, McKenna S, Lovas K, Smedstad L, Fortin P (2000) International adaptation of the Rheumatoid Arthritis Quality of Life Scale (RAQoL). Qual Life Res 9(3):342
Marra CA, Rashidi AA, Guh D, Kopec JA, Abrahamowicz M, Esdaile JM et al (2005) Are indirect utility measures reliable and responsive in rheumatoid arthritis patients? Qual Life Res 14(5):1333–1344
Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS et al (1988) The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 31(3):315–324
Fuchs HA, Brooks RH, Callahan LF, Pincus T (1989) A simplified twenty-eight-joint quantitative articular index in rheumatoid arthritis. Arthritis Rheum 32(5):531–537
Citera G, Arriola MS, Maldonado-Cocco JA, Rosemffet MG, Sanchez MM, Goni MA et al (2004) Validation and crosscultural adaptation of an Argentine Spanish version of the health assessment questionnaire disability index. J Clin Rheumatol 10(3):110–115
van der Heijde D (2000) How to read radiographs according to the Sharp/van der Heijde method. J Rheumatol 27(1):261–263
Streiner D, Norman G (2008) Health Measurement Scales: a practical guide to their development and use, 4th edn. Oxford University Press, New York
Swaine-Verdier A, Doward LC, Hagell P, Thorsen H, McKenna SP (2004) Adapting quality of life instruments. Value Health 7(Suppl 1):S27–S30
Nunnally J, Bernstein I (1994) Psychometric theory. McGraw-Hill, New York
Marra CA, Woolcott JC, Kopec JA, Shojania K, Offer R, Brazier JE et al (2005) A comparison of generic, indirect utility measures (the HUI2, HUI3, SF-6D, and the EQ-5D) and disease-specific instruments (the RAQoL and the HAQ) in rheumatoid arthritis. Soc Sci Med 60(7):1571–1582
Garip Y, Eser F, Bodur H (2010) Health-related quality of life in rheumatoid arthritis: comparison of RAQoL with other scales in terms of disease activity, severity of pain, and functional status. Rheumatol Int.
Liang MH (2000) Longitudinal construct validity: establishment of clinical meaning in patient evaluative instruments. Med Care 38(9 Suppl):II84–II90
Cohen J (1988) Statistical power analysis for the behavioral sciences, 2nd edn. Laurence Erlbaum Associates, Hillsdale
Fransen J, van Riel PL (2009) Outcome measures in inflammatory rheumatic diseases. Arthritis Res Ther 11(5):244
Landis JR, Koch GG (1977) The measurement of observer agreement for categorical data. Biometrics 33(1):159–174
Wyrwich KW, Tierney WM, Wolinsky FD (1999) Further evidence supporting an SEM-based criterion for identifying meaningful intra-individual changes in health-related quality of life. J Clin Epidemiol 52(9):861–873
McHorney CA, Tarlov AR (1995) Individual-patient monitoring in clinical practice: are available health status surveys adequate? Qual Life Res 4(4):293–307
Wolfe F, Pincus T (1994) Rheumatoid arthritis: pathogenesis, assessment, outcome, and treatment. Dekker, New York
Rasch G, Wright B (1993) Probabilistic models for some intelligence and attainment tests. MESA, Chicago
Pacheco-Tena C, Reyes-Cordero G, Mckenna SP et al (2011) Adaptation and validation of the Rheumatoid Arthritis Quality of Life Scale (RAQoL) to Mexican Spanish. Reumatol Clin 7(2):98–103
Acknowledgments
This study was supported by an unrestricted grant from Wyeth-Pfizer, Argentina.
Disclosure
Christian A. Waimann has received honoraria from Wyeth-Pfizer, Argentina for this work. All other authors declare no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
The Editor has retracted this article due to infringement of copyright. Researchers wishing to use the official RAQoL Spanish translation are requested to contact Galen Research (http://www.galenresearch.com).
An erratum to this article is available at http://dx.doi.org/10.1007/s10067-013-2397-x.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 52 kb)
Appendix
Final 30 items of the Argentinean version of the RAQoL:
-
1.
Tengo que acostarme más temprano de lo que me gustaría hacerlo.
-
2.
Me da miedo que la gente me lleve por delante o me golpee.
-
3.
Me resulta difícil encontrar calzado cómodo y que me guste.
-
4.
Evito ir a lugares con mucha gente por mi enfermedad.
-
5.
Tengo dificultades para vestirme.
-
6.
Me resulta difícil ir caminando a hacer compras.
-
7.
Las tareas de la casa me llevan más tiempo para realizarlas.
-
8.
A veces tengo dificultades para usar el inodoro.
-
9.
Frecuentemente me siento frustrada/o.
-
10.
Tengo que dejar de hacer lo que estoy haciendo para descansar un rato.
-
11.
Tengo dificultades para usar el cuchillo y el tenedor.
-
12.
Me resulta difícil concentrarme.
-
13.
Algunas veces quiero estar a solas.
-
14.
Me resulta difícil caminar grandes distancias.
-
15.
Trato de evitar darle la mano a la gente.
-
16.
Frecuentemente me siento deprimida/o.
-
17.
Me siento incapaz de compartir actividades con mis familiares o amigos.
-
18.
Tengo dificultades para bañarme.
-
19.
A veces lloro debido a mi enfermedad.
-
20.
Mi enfermedad me limita para ir a ciertos lugares.
-
21.
Me siento cansada/o, haga lo que haga.
-
22.
Siento que dependo de los demás.
-
23.
Constantemente pienso en mi enfermedad.
-
24.
Frecuentemente me enojo conmigo misma/o.
-
25.
Para mí es demasiado esfuerzo salir y encontrarme con gente.
-
26.
Duermo muy mal durante la noche.
-
27.
Me resulta difícil atender a la gente que me rodea.
-
28.
Siento que no puedo controlar mi enfermedad.
-
29.
Evito el contacto físico con otras personas.
-
30.
No puedo usar toda la ropa que me gustaría.
About this article
Cite this article
Waimann, C.A., Dal Pra, F.M., Marengo, M.F. et al. RETRACTED ARTICLE: Quality of life of patients with rheumatoid arthritis in Argentina: reliability, validity, and sensitivity to change of a Spanish version of the Rheumatoid Arthritis Quality of Life questionnaire. Clin Rheumatol 31, 1065–1071 (2012). https://doi.org/10.1007/s10067-012-1976-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-012-1976-6