Abstract
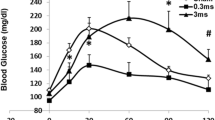
Artificial pancreas systems control insulin-mediated glucose uptake. Although these systems are widely used in the clinical setting, they are still fraught with structural and biological problems. The non-insulin mediated glucose uptake (NIMGU) mechanism could be an alternative candidate as a target system for the artificial control of peripheral glucose uptake. Although the sympathetic nervous system is known to be one of the regulators of NIMGU, the effects of peripheral sympathetic activation on glucose uptake have not been well documented. We electrically stimulated a sympathetic nerve fascicle to clarify the possibility of controlling peripheral glucose uptake. A sympathetic signal was microneurographically obtained in the unilateral sciatic nerve in normal (NRML), insulin-resistant high-fat-fed (HFF), and streptozotocin-induced insulin-depleted (STZ) rats, and electrical stimulation was applied via the microelectrode (microstimulation). The microstimulation was also applied to sites other than the sympathetic fascicles in an additional group of normal rats (NSYMP group). The stimulation applied to the sympathetic fibers resulted in an immediate and transient decrease of blood glucose (BG) in the NRML, HFF, and STZ groups, with little change in the plasma insulin. The change in BG level seemed to depend on the basal BG level (NRML < HFF < STZ). In contrast, no reduction in BG was observed in the NSYMP group. These results suggest that microstimulation in the peripheral sympathetic fascicle could enhance glucose uptake in peripheral tissues—independently of insulin function—and show an alternative possibility for controlling glucose uptake.




Similar content being viewed by others
References
Renard E, Farret A, Place J, Wojtusciszyn A, Bringer J. Towards an artificial pancreas at home. Diabetes Metab. 2011;37:S94–8.
Hoshino M, Haraguchi Y, Mizushima I, Sakai M. Recent progress in mechanical artificial pancreas. J Artif Organs. 2009;12:141–9.
Jumpertz R, Thearle MS, Bunt JC, Krakoff J. Assessment of non-insulin mediated glucose uptake: association with body weight and glycemic status. Metabolism. 2010;59:1396–401.
Ryder JW, Chibalin AV, Zierath JR. Intracellular mechanisms underlying increases in glucose uptake in response to insulin or exercise in skeletal muscle. Acta Physiol Scand. 2001;171:249–57.
Sudo M, Minokoshi Y, Shimazu T. Ventromedial hypothalamic stimulation enhances peripheral glucose uptake in anesthetized rats. Am J Physiol. 1991;261:E298–303.
Minokoshi Y, Haque MS, Shimazu T. Microinjection of leptin into the ventromedial hypothalamus increases glucose uptake in peripheral tissues in rats. Diabetes. 1999;48:287–91.
Haque MS, Minokoshi Y, Hamai M, Iwai M, Horiguchi M, Shimazu T. Role of the sympathetic nervous system and insulin in enhancing glucose uptake in peripheral tissues after intrahypothalamic injection of leptin in rats. Diabetes. 1999;48:1706–12.
Toda C, Shiuchi T, Lee S, Yamato-Esaki M, Fujino Y, Suzuki A, Okamoto S, Minokoshi Y. Distinct effects of leptin and a melanocortin receptor agonist injected into medial hypothalamic nuclei on glucose uptake in peripheral tissues. Diabetes. 2009;58:2757–65.
Peckham PH, Knutson JS. Functional electrical stimulation for neuromuscular applications. Annu Rev Biomed Eng. 2005;7:327–60.
Gordon T, Sulaiman OA, Ladak A. Chapter 24: electrical stimulation for improving nerve regeneration: where do we stand? Int Rev Neurobiol. 2009;87:433–44.
Kusunoki M, Sato D, Nakamura T. Effects of electrical micro-stimulation of peripheral sympathetic nerve on glucose metabolism in high-fat fed rats. Diabetes. 2009;58:A399.
Sato D, Nakamura T, Tsutsumi K, Shinzawa G, Karimata T, Okawa T, Feng Z, Kusunoki M. Site dependency of fatty acid composition in adipose triacylglycerol in rats and its absence as a result of high-fat feeding. Metabolism. 2012;61:92–8.
Nakamura T, Kawahara K, Kusunoki M, Feng Z. Microneurography in anesthetized rats for the measurement of sympathetic nerve activity in the sciatic nerve. J Neurosci Methods. 2003;131:35–9.
Hagbarth KE, Vallbo ÅB. Pulse and respiratory grouping of sympathetic impulses in human muscle nerves. Acta Physiol Scand. 1968;74:96–108.
Delius W, Hagbarth KE, Hongell A, Wallin BG. General characteristics of sympathetic activity in human muscle nerves. Acta Physiol Scand. 1972;84:65–81.
Vallbo ÅB, Hagbarth KE, Torebjörk HE, Wallin BG. Somatosensory, proprioceptive, and sympathetic activity in human peripheral nerves. Physiol Rev. 1979;59:919–57.
Mark AL, Victor RG, Nerhed C, Wallin BG. Microneurographic studies of the mechanisms of sympathetic nerve responses to static exercise in humans. Circ Res. 1985;57:461–9.
Anderson EA, Hoffman RP, Balon TW, Sinkey CA, Mark AL. Hyperinsulinemia produces both sympathetic neural activation and vasodilation in normal humans. J Clin Invest. 1991;87:2246–52.
Kurose T, Tsuda K, Ishida H, Tsuji K, Okamoto Y, Tsuura Y, Kato S, Usami M, Imura H, Seino Y. Glucagon, insulin and somatostatin secretion in response to sympathetic neural activation in streptozotocin-induced diabetic rats. A study with the isolated perfused rat pancreas in vitro. Diabetologia. 1992;35:1035–41.
Tsuchimochi H, Nakamoto T, Matsukawa K. Centrally evoked increase in adrenal sympathetic outflow elicits immediate secretion of adrenaline in anesthetized rats. Exp Physiol. 2010;95:93–106.
Peth JA, Kinnick TR, Youngblood EB, Tritschler HJ, Henriksen EJ. Effects of a unique conjugate of α-lipoic acid and γ-linolenic acid on insulin action in obese Zucker rats. Am J Physiol. 2000;278:R453–9.
Chang JC, Wu MC, Liu IM, Cheng JT. Increase of insulin sensitivity by stevioside in fructose-rich chow-fed rats. Horm Metab Res. 2005;37:610–6.
Barth E, Albuszies G, Baumgart K, Matejovic M, Wachter U, Vogt J, Radermacher P, Calzia E. Glucose metabolism and catecholamines. Crit Care Med. 2007;35:S508–18.
Nakamura T, Kawahara K, Kusunoki M, Hara T, Minejima I. Involvement of the nervous system in the control of glucose uptake in contracting hindlimb muscle in the rat. Horm Metab Res. 1997;29:593–8.
Tanishita T, Shimizu Y, Minokoshi Y, Shimazu T. The β3-adrenergic agonist BRL37344 increases glucose transport into L6 myosytes through a mechanism different from that of insulin. J Biochem. 1997;122:90–5.
Rybicki KJ, Kaufman MP. Stimulation of group III and IV muscle afferents reflexly decreases total pulmonary resistance in dogs. Respir Physiol. 1985;59:185–95.
Dodt C, Lönnroth P, Fehm HL, Elam M. Intraneural stimulation elicits an increase in subcutaneous interstitial glycerol levels in humans. J Physiol. 1999;521:545–52.
Lorrain J, Angel I, Duval N, Eon MT, Oblin A, Langer SZ. Adrenergic and nonadrenergic cotransmitters inhibit insulin secretion during sympathetic stimulation in dogs. Am J Physiol. 1992;263:E72–8.
McIntyre CC, Savasta M, Walter BL, Vitek JL. How does deep brain stimulation work? Present understanding and future questions. J Clin Neurophysiol. 2004;21:40–50.
Acknowledgments
We thank Akihito Sawaguchi, Kei Momotani, Minehisa Koshi, Akino Fujiyoshi, and Miyuki Kaga for their assistance in the animal experiments. This study was supported financially in part by Grants-in-Aid for Scientific Research (B) (17300139) and for the Global COE Program from the Japan Society for the Promotion of Science.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Sato, D., Shinzawa, G., Kusunoki, M. et al. Effects of electrical microstimulation of peripheral sympathetic nervous fascicle on glucose uptake in rats. J Artif Organs 16, 352–358 (2013). https://doi.org/10.1007/s10047-013-0700-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10047-013-0700-x