Abstract
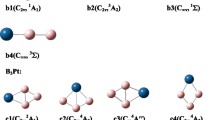
We have studied the influence of hydrogenation on the relative stability of the low-lying isomers of the anionic B −7 cluster, computationally. It is known that the pure-boron B −7 cluster has a doubly (σ- and π-) aromatic C6v (3A1) quasi-planar wheel-type triplet global minimum (structure 1), a low-lying σ-aromatic and π-antiaromatic quasi-planar singlet C2v (1A1) isomer 2 (0.7 kcal mol−1 above the global minimum), and a planar doubly (σ- and π-) antiaromatic C2v (1A1) isomer 3 (7.8 kcal mol−1 above the global minimum). However, upon hydrogenation, an inversion in the stability of the species occurs. The planar B7H −2 (C2v, 1A1) isomer 4, originated from the addition of two hydrogen atoms to the doubly antiaromatic B −7 isomer 3, becomes the global minimum structure. The second most stable B7H −2 isomer 5, originated from the quasi-planar triplet wheel isomer 1 of B −7 , was found to be 27 kcal mol−1 higher in energy. The inversion in stability occurs due to the loss of the doubly aromatic character in the wheel-type global minimum isomer (C6v, 3A1) of B −7 upon H2−addition. In contrast, the planar isomer of B −7 (C2v, 1A1) gains aromatic character upon addition of two hydrogen atoms, which makes it more stable.

The B7H2-global minimum structure and its σ-aromatic and π-antiaromatic MOs






Similar content being viewed by others
References
Hanley L, Whitten JL, Anderson SL (1988) J Phys Chem 92:5803–5812
Hanley L, Anderson SL (1987) J Phys Chem 91:5161–5163
Hanley L, Anderson SL (1988) J Chem Phys 89:2848–2860
Hintz PA, Ruatta SA, Anderson SL (1990) J Chem Phys 92:292–303
Ruatta SA, Hintz PA, Anderson SL (1991) J Chem Phys 94:2833–2847
Hintz PA, Sowa MB, Ruatta SA, Anderson SL (1991) J Chem Phys 94:6446–6458
Placa SJL, Roland PA, Wynne JJ (1992) Chem Phys Lett 190:163–168
Bonacic-Koutecky V, Fantucci P, Koutecky J (1991) Chem Rev 91:1035–1108
Kato H, Yamashita K, Morokuma K (1992) Chem Phys Lett 190:361–366
Ray AK, Howard IA, Kanal KM (1992) Phys Rev B 45:14247–14255
Boustani I (1997) Phys Rev B 55:16426–16438
Wang ZX, Schleyer PvR (2001) Science 292:2465–2469
Zhai H-J, Wang LS, Alexandrova AN, Boldyrev AI, Zakrzewski VG (2003) J Phys Chem A 107:9319–9328
Zhai H-J, Wang LS, Alexandrova AN, Boldyrev AI (2002) J Chem Phys 117:7917–7924
Alexandrova AN, Boldyrev AI, Zhai HJ, Wang LS, Sheiner E, Fowler PW (2003) J Phys Chem A 107:1359–1369
Alexandrova AN, Boldyrev AI, Zhai H-J, Wang LS (2004) J Phys Chem A 108:3509–3517
Zhai H-J, Alexandrova AN, Birch KA, Boldyrev AI, Wang LS (2003) Angew Chem Int Ed 42:6004–6008
Zhai H-J, Kiran B, Li J, Wang LS (2003) Nat Mater 2:827–833
Chandrasekhar J, Jemmis ED, Schleyer PvR (1979) Tetrahedron Lett 39:3707–3710
Martin-Santamaria S, Rzepa HS (2000) Chem Commun 16:1503–1504
Präsang C, Mlodzianowska A, Sahin Y, Hofmann M, Geiseler G, Massa W, Berndt A (2002) Angew Chem Int Ed 41:3380–3382
Präsang C, Hofmann M, Geiseler G, Massa W, Berndt A (2002) Angew Chem Int Ed 41:1526–1529
Präsang C, Mlodzianowska A, Geiseler G, Massa W, Hofmann M, Berndt A (2003) Pure Appl Chem 75:1175–1182
Amseis P, Mesbah W, Präsang C, Hofmann M, Geiseler G, Massa W, Berndt A (2003) Organometallics 22:1594–1596
Mesbah W, Präsang C, Hofmann M, Geiseler G, Massa W, Berndt A (2003) Angew Chem Int Ed 42:1717–1719
Lipscomb WN (1963) Boron hydrides. Benjamin, New York
Muetterties EL (1975) Boron hydride chemistry. Academic, New York
Cotton FA, Wilkinson G, Murillo CA, Bochmann M (1999) Advanced inorganic chemistry, 6th edn. Wiley, New York
Ricca A, Bauschlicher CW Jr (1997) J Chem Phys 106:2317–2322
Curtiss LA, Pople JA (1989) J Chem Phys 91:4809–4812
Schleyer PvR, Najafian K, Mebel A (1998) Inorg Chem 37:6765–6772
Goss JP, Briddon PR, Jones R, Teukam Z, Ballutaud D, Jomard F, Chevallier J, Bernard M, Deneuville A (2003) Phys Rev B 68:235209–235218
DiLabio GA, Matusek DR (2000) Chem Phys Lett 317:597–602
Wang P, Orimo S, Tanabe K, Fujii H (2003) J Alloys Comp 350:218–221
Boustani I (1995) Chem Phys Lett 240:135–140
Boustani I, Quandt A, Hernandez E, Rubio A (1999) J Chem Phys 110:3176–3185
Boustani I (1994) Int J Quantum Chem 52:1081–1111
Ricca A, Bauschlicher CW Jr (1996) Chem Phys 208:233–242
Kato H, Tanaka E (1991) J Comput Chem 12:1097–1107
Alexandrova AN, Boldyrev AI, Fu Y-J, Wang X-B, Wang L-S (2004) J Chem Phys 121:5709–5719
Hartke B (1993) J Phys Chem 97:9973–9976
Deaven DM, Ho KM (1995) Phys Rev Lett 75:288–291
Parr RG, Yang W (1989) Density-functional theory of atoms and molecules. Oxford University Press, Oxford
Becke AD (1993) J Chem Phys 98:5648–5652
Perdew JP, Chevary JA, Vosko SH, Jackson KA, Pederson MR, Singh DJ, Fiolhais C (1992) Phys Rev B 46:6671–6687
Frisch MJ, Trucks GM, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Montgomery JA, Vreven T, Kudin KN, Burant JC, Millam JM, Iyengar SS, Tomasi J, Barone V, Mennucci B, Cossi M, Scalmani G, Rega N, Petersson GA, Nakatsuji H, Kitao O, Nakai H, Klene M, Li X, Knox JE, Hratchian HP, Cross JB, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Ayala PY, Morokuma K, Voth GA, Salvador P, Dannenberg JJ, Zakrzewski VG, Dapprich S, Daniels AD, Strain MC, Farkas O, Malick DK, Rabuck AD, Raghavachari K, Foresman JB, Ortiz JV, Cui Q, Baboul AG, Clifford S, Cioslowski J, Stefanov BB, Liu A, Liashenko A, Piskorz P, Komaromi I, Martin RL, Fox DJ, Keith T, Al-Laham MA, Peng CY, Nanayakkara A, Challacombe M, Gill PMW, Johnson BG, Chen W, Wang MW, Gonzales C, Pople JA (2003) Gaussian 03, Revision A.1. Gaussian Inc., Pittsburgh
Krishnan R, Binkley JS, Seeger R, Pople JA (1980) J Chem Phys 72:650–654
Cizek J (1969) Adv Chem Phys 14:35–89
Knowles PJ, Hampel C, Werner H-J (1993) J Chem Phys 99:5219–5227
Clark T, Chandrasekhar J, Spitznagel GW, Schleyer PvR (1983) J Comput Chem 4:294–301
Frisch MJ, Pople JA, Binkley JS (1984) J Chem Phys 80:3265–3269
Hegarty D, Robb MA (1979) Mol Phys 38:1795–1812
Eade RHE, Robb MA (1981) Chem Phys Lett 83:362–368
Schlegel HB, Robb MA (1982) Chem Phys Lett 93:43–46
Bernardi F, Bottini A, McDouglas JJW, Robb MA, Schlegel HB (1984) Far Symp Chem Soc 19:137–147
Yamamoto N, Vreven T, Robb MA, Frisch MJ, Schlegel MA (1996) Chem Phys Lett 250:373–378
Frisch MJ, Ragazos IN, Robb MA, Schlegel HB (1992) Chem Phys Lett 189:524–528
Carpenter JE, Weinhold F (1988) J Mol Struct (Theochem) 169:41–62
Carpenter JE (1987) PhD Thesis, University of Wisconsin, Madison
Foster JP, Weinhold F (1980) J Am Chem Soc 102:7211–7218
Reed AE, Weinhold F (1983) J Chem Phys 78:4066–4073
Reed AE, Curtiss LA, Weinhold F (1988) Chem Rev 88:899–926
Schaftenaar G (1998) MOLDEN 3.4. CAOS/CAMM Center, The Netherlands
Acknowledgments
This work was supported partially by the donors of The Petroleum Research Fund (ACS-PRF# 38242-AC6), administered by the American Chemical Society, partially by the National Science Foundation (CHE-0404937), and partially by the Summer Research Institute at the Pacific Northwest National Laboratory operated by Battelle, Richland, Washington, USA.
Author information
Authors and Affiliations
Corresponding author
Additional information
Dedicated to Professor Dr. Paul von Ragué Schleyer on the occasion of his 75th birthday.
Rights and permissions
About this article
Cite this article
Alexandrova, A.N., Koyle, E. & Boldyrev, A.I. Theoretical study of hydrogenation of the doubly aromatic B −7 cluster. J Mol Model 12, 569–576 (2006). https://doi.org/10.1007/s00894-005-0035-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00894-005-0035-5