Abstract
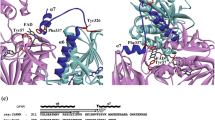
The diiron cluster-containing oxygenase CmlI catalyzes the conversion of the aromatic amine precursor of chloramphenicol to the nitroaromatic moiety of the active antibiotic. The X-ray crystal structures of the fully active, N-terminally truncated CmlIΔ33 in the chemically reduced Fe2+/Fe2+ state and a cis μ-1,2(η 1:η 1)-peroxo complex are presented. These structures allow comparison with the homologous arylamine oxygenase AurF as well as other types of diiron cluster-containing oxygenases. The structural model of CmlIΔ33 crystallized at pH 6.8 lacks the oxo-bridge apparent from the enzyme optical spectrum in solution at higher pH. In its place, residue E236 forms a μ-1,3(η 1:η 2) bridge between the irons in both models. This orientation of E236 stabilizes a helical region near the cluster which closes the active site to substrate binding in contrast to the open site found for AurF. A very similar closed structure was observed for the inactive dimanganese form of AurF. The observation of this same structure in different arylamine oxygenases may indicate that there are two structural states that are involved in regulation of the catalytic cycle. Both the structural studies and single crystal optical spectra indicate that the observed cis μ-1,2(η 1:η 1)-peroxo complex differs from the μ-η 1:η 2-peroxo proposed from spectroscopic studies of a reactive intermediate formed in solution by addition of O2 to diferrous CmlI. It is proposed that the structural changes required to open the active site also drive conversion of the µ-1,2-peroxo species to the reactive form.










Similar content being viewed by others
Abbreviations
- CmlI:
-
Full length wild-type CmlI enzyme
- CmlIΔ33:
-
33 amino acid N-terminally truncated variant of CmlI used for crystallization
- CAM:
-
Chloramphenicol
- NH2-CAM:
-
d-threo-1-(4-aminophenyl)-2-dichloroacetylamino-1,3-propanediol, the arylamine precursor of CAM and natural CmlI substrate
- Fe-AurF:
-
The functional diiron variant of the aureothin arylamine oxygenase, AurF
- Mn-AurF:
-
The catalytically inactive dimanganese variant of AurF
- P :
-
Diferric peroxo intermediate of CmlI or CmlIΔ33
- RNR-R2:
-
The R2 subunit of ribonucleotide reductase which houses the diiron cluster
- MMOH:
-
Hydroxylase component of the soluble form of methane monooxygenase, MMO
- T4moH:
-
Toluene 4-monooxygenase hydroxylase
References
Winkler R, Hertweck C (2007) ChemBioChem 8:973–977
Ju K-S, Parales RE (2010) Microbiol Mol Biol Rev 74:250–272
Kirner S, Hammer PE, Hill DS, Altmann A, Fischer I, Weislo LJ, Lanahan M, van Pée K-H, Ligon JM (1998) J Bacteriol 180:1939–1943
He J, Magarvey N, Piraee M, Vining LC (2001) Microbiology 147:2817–2829
He J, Hertweck C (2003) Chem Biol 10:1225–1232
Lee J, Simurdiak M, Zhao H (2005) J Biol Chem 280:36719–36727
Simurdiak M, Lee J, Zhao H (2006) ChemBioChem 7:1169–1172
Lu HG, Chanco E, Zhao HM (2012) Tetrahedron 68:7651–7654
Doull J, Ahmed Z, Stuttard C, Vining LC (1985) J Gen Microbiol 131:97–104
Makris TM, Chakrabarti M, Münck E, Lipscomb JD (2010) Proc Natl Acad Sci USA 107:15391–15396
Fischbach MA, Walsh CT (2006) Chem Rev 106:3468–3496
Walsh CT, Chen H, Keating TA, Hubbard BK, Losey HC, Luo L, Marshall CG, Miller DA, Patel HM (2001) Curr Opin Chem Biol 5:525–534
Pacholec M, Sello JK, Walsh CT, Thomas MG (2007) Org Biomol Chem 5:1692–1694
Piraee M, White RL, Vining LC (2004) Microbiology 150:85–94
Makris TM, Vu VV, Meier KK, Komor AJ, Rivard BS, Münck E, Que L Jr, Lipscomb JD (2015) J Am Chem Soc 137:1608–1617
Wallar BJ, Lipscomb JD (1996) Chem Rev 96:2625–2657
Fox BG, Froland WA, Dege JE, Lipscomb JD (1989) J Biol Chem 264:10023–10033
Rosenzweig AC, Frederick CA, Lippard SJ, Nordlund P (1993) Nature 366:537–543
Nordlund P, Sjöberg BM, Eklund H (1990) Nature 345:593–598
Fox BG, Shanklin J, Somerville C, Münck E (1993) Proc Natl Acad Sci USA 90:2486–2490
Lindqvist Y, Huang WJ, Schneider G, Shanklin J (1996) EMBO 15:4081–4092
Guy JE, Whittle E, Kumaran D, Lindqvist Y, Shanklin J (2007) J Biol Chem 282:19863–19871
Schirmer A, Rude MA, Li X, Popova E, del Cardayre SB (2010) Science 329:559–562
Li N, Chang W-C, Warui DM, Booker SJ, Krebs C, Bollinger JM Jr (2012) Biochemistry 51:7908–7916
Pikus JD, Studts JM, Achim C, Kauffmann KE, Münck E, Steffan RJ, McClay K, Fox BG (1996) Biochemistry 35:9106–9119
Sazinsky MH, Bard J, Di Donato A, Lippard SJ (2004) J Biol Chem 279:30600–30610
Andrews SC (2010) Biochim Biophys Acta 1800:691–705
Skulan AJ, Brunold TC, Baldwin J, Saleh L, Bollinger JM Jr, Solomon EI (2004) J Am Chem Soc 126:8842–8855
Srnec M, Rokob TA, Schwartz JK, Kwak Y, Rulisek L, Solomon EI (2012) Inorg Chem 51:2806–2820
Liu KE, Valentine AM, Qiu D, Edmondson DE, Appelman EH, Spiro TG, Lippard SJ (1995) J Am Chem Soc 117:4997–4998
Broadwater JA, Ai J, Loehr TM, Sanders-Loehr J, Fox BG (1998) Biochemistry 37:14664–14671
Yun D, Garcia-Serres R, Chicalese BM, An YH, Huynh BH, Bollinger JM Jr (2007) Biochemistry 46:1925–1932
Vu VV, Emerson JP, Martinho M, Kim YS, Münck E, Park MH, Que L Jr (2009) Proc Natl Acad Sci USA 106:14814–14819
Korboukh VK, Li N, Barr EW, Bollinger JM Jr, Krebs C (2009) J Am Chem Soc 131:13608–13609
Li N, Korboukh VK, Krebs C, Bollinger JM Jr (2010) Proc Natl Acad Sci USA 107:15722–15727
Zocher G, Winkler R, Hertweck C, Schulz GE (2007) J Mol Biol 373:65–74
Choi YS, Zhang H, Brunzelle JS, Nair SK, Zhao H (2008) Proc Natl Acad Sci USA 105:6858–6863
Winkler R, Zocher G, Richter I, Friedrich T, Schulz GE, Hertweck C (2007) Angew Chem Int Ed 46:8605–8608
McCoy AJ, Grosse-Kunstleve RW, Adams PD, Winn MD, Storoni LC, Read RJ (2007) J Appl Crystallogr 40:658–674
Emsley P, Cowtan K (2004) Acta Crystallogr E60:2126–2132
Murshudov GN, Skubak P, Lebedev AA, Pannu NS, Steiner RA, Nicholls RA, Winn MD, Longa F, Vagin AA (2011) Acta Cryst D67:355–367
Holmes MA, Le Trong I, Turley S, Sieker LC, Stenkamp RE (1991) J Mol Biol 218:583–593
Makris TM, Knoot CJ, Wilmot CM, Lipscomb JD (2013) Biochemistry 52:6662–6671
Ookubo T, Sugimoto H, Nagayama T, Masuda H, Sato T, Tanaka K, Maeda Y, Okawa H, Hayashi Y, Uehara A, Suzuki M (1996) J Am Chem Soc 118:701–702
Kim K, Lippard SJ (1996) J Am Chem Soc 118:4914–4915
Zhang X, Furutachi H, Fujinami S, Nagatomo S, Maeda Y, Watanabe Y, Kitagawa T, Suzuki M (2005) J Am Chem Soc 127:826–827
Bailey LJ, Fox BG (2009) Biochemistry 48:8932–8939
Han Z, Sakai N, Boettger LH, Klinke S, Hauber J, Trautwein AX, Hilgenfeld R (2015) Structure 23:882–892
Fiedler AT, Shan X, Mehn MP, Kaizer J, Torelli S, Frisch JR, Kodera M, Que L Jr (2008) J Phys Chem A 112:13037–13044
Ray WJ, Puvathingal JM (1985) Anal Biochem 146:307–312
Xiong J, Kurtz DM Jr, Ai J, Sanders-Loehr J (2000) Biochemistry 39:5117–5125
Wei P-P, Skulan AJ, Mitić N, Yang Y-S, Saleh L, Bollinger JM Jr, Solomon EI (2004) J Am Chem Soc 126:3777–3788
Banerjee R, Meier KK, Münck E, Lipscomb JD (2013) Biochemistry 52:4331–4342
Rosenzweig AC, Nordlund P, Takahara PM, Frederick CA, Lippard SJ (1995) Chem Biol 2:409–418
Voegtli WC, Khidekel N, Baldwin J, Ley BA, Bollinger JM Jr, Rosenzweig AC (2000) J Am Chem Soc 122:3255–3261
Tinberg CE, Lippard SJ (2011) Acc Chem Res 44:280–288
Kovaleva EG, Lipscomb JD (2007) Science 316:453–457
Kovaleva EG, Lipscomb JD (2008) Biochemistry 47:11168–11170
Knoot CJ, Purpero VM, Lipscomb JD (2015) Proc Natl Acad Sci USA 112:388–393
Wallar BJ, Lipscomb JD (2001) Biochemistry 40:2220–2233
Mitić N, Schwartz JK, Brazeau BJ, Lipscomb JD, Solomon EI (2008) Biochemistry 47:8386–8397
Yang Y-S, Broadwater JA, Pulver SC, Fox BG, Solomon EI (1999) J Am Chem Soc 121:2770–2783
Schwartz JK, Wei P-P, Mitchell KH, Fox BG, Solomon EI (2008) J Am Chem Soc 130:7098–7109
Liu Y, Nesheim JC, Lee S-K, Lipscomb JD (1995) J Biol Chem 270:24662–24665
Logan DT, Su XD, Aberg A, Regnstrom K, Hajdu J, Eklund H, Nordlund P (1996) Structure 4:1053–1064
Elango N, Radhakrishnan R, Froland WA, Wallar BJ, Earhart CA, Lipscomb JD, Ohlendorf DH (1997) Protein Sci 6:556–568
Banerjee R, Proshlyakov Y, Lipscomb JD, Proshlyakov DA (2015) Nature 518:431–434
Lee SK, Lipscomb JD (1999) Biochemistry 38:4423–4432
Acknowledgments
This work was supported by the National Institutes of Health Grants GM 100943 and GM 118030 (to J.D.L.) and NIH graduate traineeship GM 08700 (to C. J. K.). We would like to thank Carrie Wilmot and her research group for many helpful discussions. We also thank Ed Hoeffner at the University of Minnesota Kahlert Structural Biology Laboratory for suggestions in indexing the CmlIΔ33 data and collecting the data sets. We also thank Klaus Lovendahl for generating the expression construct for the truncated enzyme and Anna Komor and Brent Rivard for assistance with biochemical characterization. Diffraction data were collected at Argonne National Laboratory, Structural Biology Center Beamline 19-ID at the Advanced Photon Source. Argonne is operated by UChicago Argonne, LLC, for the US Department of Energy, Office of Biological and Environmental Research under contract DE-AC02-06CH11357. We are thankful for computational resources from the Supercomputing Institute and the facilities at the Kahlert Structural Biology Laboratory at the University of Minnesota.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Knoot, C.J., Kovaleva, E.G. & Lipscomb, J.D. Crystal structure of CmlI, the arylamine oxygenase from the chloramphenicol biosynthetic pathway. J Biol Inorg Chem 21, 589–603 (2016). https://doi.org/10.1007/s00775-016-1363-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00775-016-1363-x