Abstract
A new type of carbon ceramic electrode modified with bismuth oxide nanoparticles (referred to as Bi-CCE) was fabricated via the sol-gel method. The Bi-CCE was applied to the determination of syringic acid by square wave adsorptive stripping voltammetry. The electrochemical properties of the Bi-CCE and the voltammetric response to syringic acid were investigated by cyclic voltammetry. The effects of the pH value of supporting electrolyte, of accumulation potential, accumulation time, SW mode parameters, and of possible interferents were tested. Under optimized conditions, the oxidation peak current (best measured at 0.8 V vs Ag/AgCl after an accumulation time of 20 s) increases linearly in the 0.4 to 24 μmol·L−1 syringic acid concentration range. Other figures of merit include an LOD of 47 nmol·L−1, a sensitivity of 3.3 μA·μmol−1·L·cm−2, and a relative standard deviation of 4.7% (for n = 5) at 2 μmol·L−1 of syringic acid. The method was successfully applied to the determination of syringic acid in red, white and rose wine as well as water samples.

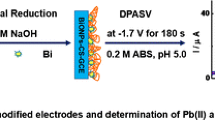
Schematic presentation of the preparation of carbon ceramic electrode (CCE) containing Bi2O3 nanoparticles and its application in square wave adsorptive stripping voltammetric (SW AdSV) determination of syringic acid.






Similar content being viewed by others
References
Wang J, Lu J, Hocoeevar SB, Farias PAM, Ogorevc B (2000) Bismuth-coated carbon electrodes for anodic stripping voltammetry. Anal Chem 72(14):3218–3222
Królicka A, Bobrowski A (2004) Bismuth film electrode for adsorptive stripping voltammetry – electrochemical and microscopic study. Electrochem Commun 6(2):99–104
Švancara I, Prior C, Hočevar SB, Wang J (2010) A decade with bismuth–based electrodes in electroanalysis. Electroanalysis 22(13):1405–1420
Guzsvány V, Kádár M, Gaál F, Bjelica L, Tóth K (2006) Bismuth film electrode for the cathodic electrochemical determination of thiamethoxam. Electroanalysis 18(13–14):1363–1371
Guzsvány V, Kádár M, Papp Z, Bjelica L, Gaál F, Tóth K (2008) Monitoring of photocatalytic degradation of selected neonicotinoid insecticides by cathodic voltammetry with a bismuth film electrode. Electroanalysis 20(3):291–300
Baś B, Węgiel K, Jedlińska K (2015) The renewable bismuth bulk annular band working electrode: fabrication and application in the adsorptive stripping voltammetric determination of nickel(II) and cobalt(II). Anal Chim Acta 881:44–53
Armstrong KC, Tatum CE, Dansby-Sparks RN, Chambers JQ, Xue ZL (2010) Individual and simultaneous determination of lead, cadmium, and zinc by anodic stripping voltammetry at a bismuth bulk electrode. Talanta 82(2):675–680
Baś B, Węgiel K, Jedlińska K (2015) New voltammetric sensor based on the renewable bismuth bulk annular band electrode and its application for the determination of palladium(II). Electrochim Acta 178:665–672
Węgiel K, Jedlińska K, Baś B (2016) Application of bismuth bulk annular band electrode for determination of ultratrace concentrations of thallium(I) using stripping voltammetry. J Hazard Mater 310:199–206
Węgiel K, Grabarczyk M, Kubiak WW, Baś B (2017) A reliable and sensitive Voltammetric determination of Mo(VI) at the in situ renovated bismuth bulk annular band electrode. J Electrochem Soc 164(6):352–357
Węgiel K, Robak J, Baś B (2017) Voltammetric determination of iron with catalytic system at a bismuth bulk annular band electrode electrochemically activated. RSC Adv 7:22027–22033
Tall OE, Beh D, Jaffrezic-Renault N, Vittori O (2010) Electroanalysis of some nitro-compounds using bulk bismuth electrode. Int J Environ Analyt Chem 90(1):40–48
Prchal V, Ottenschlagerova A, Vyskocil V, Barek J (2016) Voltammetric determination of 5-nitroindazole using a bismuth bulk electrode. Anal Lett 49(1):49–55
Węgiel K, Baś B (2017) Voltammetric characteristics and determination of clothianidin using a bismuth bulk annular band electrode regenerated in situ. Ionics. https://doi.org/10.1007/s11581-017-2105-y
Królicka A, Pauliukaite R, Švancara I, Metelka R, Bobrowski A, Norkus E, Kalcher K, Vytřas K (2002) Bismuth-film-plated carbon paste electrodes. Electrochem Commun 4(2):193–196
Yang D, Wang L, Chen Z, Megharaj M, Naidu R (2014) Anodic stripping voltammetric determination of traces of Pb(II) and Cd(II) using a glassy carbon electrode modified with bismuth nanoparticles. Microchim Acta 181(11–12):1199–1206
Kruusma J, Banks CE, Compton RG (2004) Mercury–free sono–electroanalytical detection of lead in human blood by use of bismuth–film–modified boron–doped diamond electrodes. Anal Bioanal Chem 379(4):700–706
Niu P, Fernández-Sánchez C, Gich M, Navarro-Hernández C, Fanjul-Bolado P, Roig A (2016) Screen-printed electrodes made of a bismuth nanoparticle porous carbon nanocomposite applied to the determination of heavy metal ions. Microchim Acta 183(2):617–623
Li Y, Sun G, Zhang Y, Ge C, Bao N, Wang Y (2014) A glassy carbon electrode modified with bismuth nanotubes in a silsesquioxane framework for sensing of trace lead and cadmium by stripping voltammetry. Microchim Acta 181(7–8):751–757
Hu X, Pan D, Lin M, Han H, Li F (2016) Graphene oxide-assisted synthesis of bismuth nanosheets for catalytic stripping voltammetric determination of iron in coastal waters. Microchim Acta 183(2):855–861
Stoytcheva M, Zlatev R, Montero G, Velkova Z, Gochev V (2017) Nanostructured platform for the sensitive determination of paraoxon by using an electrode modified with a film of graphite-immobilized bismuth. Microchim Acta 184(8):2707–2714
Liu L, Ma Z, Zhu X, Alshahrani LA, Tie S, Nan J (2016) A glassy carbon electrode modified with carbon nano-fragments and bismuth oxide for electrochemical analysis of trace catechol in the presence of high concentrations of hydroquinone. Microchim Acta 183(12):3293–3301
Devasenathipathy R, Karthik R, Chen SM, Ali MA, Mani V, Lou BS, Al-Hemaid FMA (2015) Enzymatic glucose biosensor based on bismuth nanoribbons electrochemically deposited on reduced graphene oxide. Microchim Acta 182(13–14):2165–2172
Cerovac S, Guzsvány V, Email Kónya Z, Ashrafi AM, Švancara I, Rončević S, Kukovecz Á, Dalmacija B, Vytřas K (2015) Trace level voltammetric determination of lead and cadmium in sediment pore water by a bismuth-oxychloride particle-multiwalled carbon nanotube composite modified glassy carbon electrode. Talanta 134:640–649
Zheng J, Sheng Q, Li L, Shen Y (2007) Bismuth hexacyanoferrate–modified carbon ceramic electrodes prepared by electrochemical deposition and its electrocatalytic activity towards oxidation of hydrazine. J Electroanal Chem 611(1–2):155–161
Robak J, Burnat B, Leniart A, Kisielewska A, Brycht M, Skrzypek S (2016) The effect of carbon material on the electroanalytical determination of 4-chloro-3-methylphenol using the sol-gel derived carbon ceramic electrodes. Sensor Actuat B-Chem 236:318–325
Arribas AS, Martinez-Fernandez M, Chicharro M (2012) The role of electroanalytical techniques in analysis of polyphenols in wine. Track-Trend Anal Chem 34:78–96
Kim KH, Tsao R, Yang R, Cui SW (2005) Phenolic acid profiles and antioxidant activities of wheat bran extracts and the effect of hydrolysis conditions. Food Chem 95(3):466–473
Kampa M, Alexaki VI, Notas G, Nifli AP, Nistikaki A, Hatzoglou A, Bakogeorgou E, Kouimtzoglou E, Blekas G, Boskou D, Gravanic A, Castanas E (2004) Antiproliferative and apoptotic effects of selective phenolic acids on T47D human breast cancer cells: potential mechanisms of action. Breast Cancer Res 6:63–74
Huang Y, Lu W, Chen B, Wu M, Li S (2015) Determination of 13 phenolic compounds in Rice wine by high–performance liquid chromatography. Food Anal Methods 8(4):825–832
Martelo-Vidal MJ, Vázquez M (2014) Determination of polyphenolic compounds of red wines by UV-VIS-NIR spectroscopy and chemometrics tools. Food Chem 158:28–34
Ciepiela F, Sordoń W, Jakubowska M (2015) Principal components – based techniques in Voltammetric determination of Caffeic, Syringic and Vanillic acids. Electroanalysis 28(3):54–554
Sordoń W, Salachna A, Jakubowska M (2016) Voltammetric determination of caffeic, syringic and vanillic acids taking into account uncertainties in both axes. J Electroanal Chem 764:23–30
Tslonsky M, Gun G, Glezer V, Lev O (1994) Sol–gel derived ceramic–carbon composite electrodes: introduction and scope applications. Anal Chem 66(10):1747–1753
Sun W, Xi M, Zhang L, Zhan T, Gao H, Jiao K (2010) Electrochemical behaviors of thymine on a new ionic liquid modified carbon electrode and its detection. Electrochim Acta 56(1):222–226
Petković BB, Stanković D, Milčić M, Sovilj SP, Manojlović D (2014) Dinuclear copper(II) octaazamacrocyclic complex in a PVC coated GCE and graphite as a voltammetric sensor for determination of gallic acid and antioxidant capacity of wine samples. Talanta 132:513–519
Acknowledgements
The authors are grateful to Prof. Bogusław Baś from AGH University of Science and Technology in Cracow for help in preparation of the electrodes and for his very helpful hints.
This work was supported by the Polish National Science Centre (Project No. 2015/19/B/ST5/01380).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author(s) declare that they have no competing interests.
Electronic supplementary material
ESM 1
(DOCX 288 kb)
Rights and permissions
About this article
Cite this article
Robak, J., Węgiel, K., Burnat, B. et al. A carbon ceramic electrode modified with bismuth oxide nanoparticles for determination of syringic acid by stripping voltammetry. Microchim Acta 184, 4579–4586 (2017). https://doi.org/10.1007/s00604-017-2504-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-017-2504-9