Abstract
Background
Serine protease inhibitor Kazal type 1 (SPINK1) is expressed in normal human pancreatic acinar cells and in a variety of tumors, and binds to the epidermal growth factor receptor (EGFR), mediating cell proliferation through the mitogen-activated protein kinase cascade in pancreatic cancer cell lines. Here, we aimed to assess SPINK1 and EGFR expression in various neoplastic lesions, including tissues demonstrating precancerous changes.
Methods
Surgical specimens of pancreatic ductal adenocarcinoma (n = 23), intraductal papillary mucinous neoplasm (IPMN; n = 21), pancreatic neoplasms other than ductal adenocarcinoma (n = 8), chronic pancreatitis (n = 11), and pancreatic intraepithelial neoplasia (PanIN) lesions within the resected specimens were analyzed immunohistochemically for SPINK1 and EGFR expression.
Results
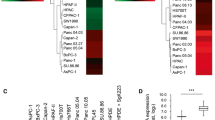
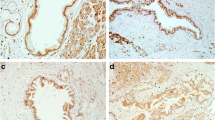
Sixty-five PanIN-1A, 32 PanIN-1B, 17 PanIN-2, and 6 PanIN-3 were identified. Both SPINK1 and EGFR were expressed in almost all PanIN lesions. All tubular ductal adenocarcinoma, IPMN, and mucinous cystadenocarcinoma samples (neoplasms of ductal origin) expressed SPINK1, whereas acinar cell carcinoma, anaplastic carcinoma, adenosquamous carcinoma, insulinoma, and islet cell carcinoma did not. EGFR was expressed in 87 % of tubular adenocarcinoma and 48 % of IPMN lesions. Among IPMN lesions, malignant lesions (IPMC) expressed EGFR more often than benign lesions (IPMA) did. Scattered expression of EGFR was observed in normal pancreatic ducts and within the tubular complex within chronic pancreatitis lesions.
Conclusions
These results indicate that SPINK1 plays a role as a growth factor, signaling through the EGFR pathway in pancreatic ductal adenocarcinoma and neoplasms, and that the EGFR is involved in the malignant transformation of IPMN.



Similar content being viewed by others
References
Ohmuraya M, Ozaki N, Hirota M, Baba H, Yamamura K. Serine protease inhibitor Kazal type 1 (SPINK1): beyond the trypsin inhibitor. Curr Enzyme Inhib. 2009;5:110–6.
Kazal LA, Spicer DS, Brahinsky RA. Isolation of a crystalline trypsin inhibitor-anticoagulant protein from the pancreas. J Am Chem Soc. 1948;70:3034–40.
Horii A, Kobayashi T, Tomita N, Yamamoto T, Fukushige S, Murotsu T, et al. Primary structure of human pancreatic secretory trypsin inhibitor (PSTI) gene. Biochem Biophys Res Commun. 1987;149(2):635–41. pii: 0006-291X(87)90415-3.
Bartelt DC, Shapanka R, Greene LJ. The primary structure of the human pancreatic secretory trypsin inhibitor. Amino acid sequence of the reduced S-aminoethylated protein. Arch Biochem Biophys. 1977;179(1):189–99.
Ohmuraya M, Hirota M, Araki K, Baba H, Yamamura K. Enhanced trypsin activity in pancreatic acinar cells deficient for serine protease inhibitor kazal type 3. Pancreas. 2006;33(1):104–6. doi:10.1097/01.mpa.0000226889.86322.9b.
Ohmuraya M, Hirota M, Araki M, Mizushima N, Matsui M, Mizumoto T, et al. Autophagic cell death of pancreatic acinar cells in serine protease inhibitor Kazal type 3-deficient mice. Gastroenterology. 2005;129(2):696–705. doi:10.1016/j.gastro.2005.05.057.
Paju A, Stenman UH. Biochemistry and clinical role of trypsinogens and pancreatic secretory trypsin inhibitor. Crit Rev Clin Lab Sci. 2006;43(2):103–42. doi:10.1080/10408360500523852.
Fukayama M, Hayashi Y, Koike M, Ogawa M, Kosaki G. Immunohistochemical localization of pancreatic secretory trypsin inhibitor in fetal and adult pancreatic and extrapancreatic tissues. J Histochem Cytochem. 1986;34(2):227–35.
Matsuda K, Ogawa M, Murata A, Kitahara T, Kosaki G. Elevation of serum immunoreactive pancreatic secretory trypsin inhibitor contents in various malignant diseases. Res Commun Chem Pathol Pharmacol. 1983;40(2):301–5.
Shibata T, Ogawa M, Takata N, Matsuda K, Niinobu T, Uda K, et al. Distribution of pancreatic secretory trypsin inhibitor in various human tissues and its inactivation in the gastric mucosa. Res Commun Chem Pathol Pharmacol. 1987;55(2):243–8.
Kitahara T, Takatsuka Y, Fujimoto KI, Tanaka S, Ogawa M, Kosaki G. Radioimmunoassay for human pancreatic secretory trypsin inhibitor: measurement of serum pancreatic secretory trypsin inhibitor in normal subjects and subjects with pancreatic diseases. Clin Chim Acta. 1980;103(2):135–43.
Matsuda K, Ogawa M, Shibata T, Nishibe S, Miyauchi K, Matsuda Y, et al. Postoperative elevation of serum pancreatic secretory trypsin inhibitor. Am J Gastroenterol. 1985;80(9):694–8.
Wang J, Ohmuraya M, Hirota M, Baba H, Zhao G, Takeya M, et al. Expression pattern of serine protease inhibitor kazal type 3 (Spink3) during mouse embryonic development. Histochem Cell Biol. 2008. doi:10.1007/s00418-008-0425-8.
Huhtala ML, Kahanpaa K, Seppala M, Halila H, Stenman UH. Excretion of a tumor-associated trypsin inhibitor (TATI) in urine of patients with gynecological malignancy. Int J Cancer. 1983;31(6):711–4.
Murata A, Ogawa M, Uda K, Matsuura N, Watanabe Y, Baba T, et al. Release of pancreatic secretory trypsin inhibitor from human hepatoblastoma cells on stimulation with cytokines. Life Sci. 1988;43(15):1233–40.
Ogata N. Demonstration of pancreatic secretory trypsin inhibitor in serum-free culture medium conditioned by the human pancreatic carcinoma cell line CAPAN-1. J Biol Chem. 1988;263(26):13427–31.
Ogawa M, Matsuura N, Higashiyama K, Mori T. Expression of pancreatic secretory trypsin inhibitor in various cancer cells. Res Commun Chem Pathol Pharmacol. 1987;55(1):137–40.
Stenman UH, Koivunen E, Itkonen O. Biology and function of tumor-associated trypsin inhibitor, TATI. Scand J Clin Lab Investig Suppl. 1991;207:5–9.
Stenman UH, Huhtala ML, Koistinen R, Seppala M. Immunochemical demonstration of an ovarian cancer-associated urinary peptide. Int J Cancer. 1982;30(1):53–7.
Huhtala ML, Pesonen K, Kalkkinen N, Stenman UH. Purification and characterization of a tumor-associated trypsin inhibitor from the urine of a patient with ovarian cancer. J Biol Chem. 1982;257(22):13713–6.
Lee YC, Pan HW, Peng SY, Lai PL, Kuo WS, Ou YH, et al. Overexpression of tumour-associated trypsin inhibitor (TATI) enhances tumour growth and is associated with portal vein invasion, early recurrence and a stage-independent prognostic factor of hepatocellular carcinoma. Eur J Cancer. 2007;43(4):736–44. doi:10.1016/j.ejca.2006.11.020.
Stenman UH. Tumor-associated trypsin inhibitor. Clin Chem. 2002;48(8):1206–9.
Tomlins SA, Rhodes DR, Yu J, Varambally S, Mehra R, Perner S, et al. The role of SPINK1 in ETS rearrangement-negative prostate cancers. Cancer Cell. 2008;13(6):519–28. doi:10.1016/j.ccr.2008.04.016.
Tonouchi A, Ohtsuka M, Ito H, Kimura F, Shimizu H, Kato M, et al. Relationship between pancreatic secretory trypsin inhibitor and early recurrence of intrahepatic cholangiocarcinoma following surgical resection. Am J Gastroenterol. 2006;101(7):1601–10. doi:10.1111/j.1572-0241.2006.00612.x.
Hunt LT, Barker WC, Dayhoff MO. Epidermal growth factor: internal duplication and probable relationship to pancreatic secretory trypsin inhibitor. Biochem Biophys Res Commun. 1974;60(3):1020–8. pii: 0006-291X(74)90415-X.
Scheving LA. Primary amino acid sequence similarity between human epidermal growth factor-urogastrone, human pancreatic secretory trypsin inhibitor, and members of porcine secretin family. Arch Biochem Biophys. 1983;226(2):411–3. pii: 0003-9861(83)90309-0.
Ozaki N, Ohmuraya M, Hirota M, Ida S, Wang J, Takamori H, et al. Serine protease inhibitor Kazal type 1 promotes proliferation of pancreatic cancer cells through the epidermal growth factor receptor. Mol Cancer Res MCR. 2009;7(9):1572–81. doi:10.1158/1541-7786.MCR-08-0567.
Hruban RH, Takaori K, Klimstra DS, Adsay NV, Albores-Saavedra J, Biankin AV, et al. An illustrated consensus on the classification of pancreatic intraepithelial neoplasia and intraductal papillary mucinous neoplasms. Am J Surg Pathol. 2004;28(8):977–87. pii: 00000478-200408000-00001.
Oikawa T, Hitomi J, Kono A, Kaneko E, Yamaguchi K. Frequent expression of genes for receptor tyrosine kinases and their ligands in human pancreatic cancer cells. Int J Pancreatol. 1995;18(1):15–23.
Korc M, Friess H, Yamanaka Y, Kobrin MS, Buchler M, Beger HG. Chronic pancreatitis is associated with increased concentrations of epidermal growth factor receptor, transforming growth factor alpha, and phospholipase C gamma. Gut. 1994;35(10):1468–73.
Barton CM, Hall PA, Hughes CM, Gullick WJ, Lemoine NR. Transforming growth factor alpha and epidermal growth factor in human pancreatic cancer. J Pathol. 1991;163(2):111–6. doi:10.1002/path.1711630206.
Friess H, Berberat P, Schilling M, Kunz J, Korc M, Buchler MW. Pancreatic cancer: the potential clinical relevance of alterations in growth factors and their receptors. J Mol Med. 1996;74(1):35–42.
Korc M, Chandrasekar B, Yamanaka Y, Friess H, Buchier M, Beger HG. Overexpression of the epidermal growth factor receptor in human pancreatic cancer is associated with concomitant increases in the levels of epidermal growth factor and transforming growth factor alpha. J Clin Investig. 1992;90(4):1352–60. doi:10.1172/JCI116001.
Overholser JP, Prewett MC, Hooper AT, Waksal HW, Hicklin DJ. Epidermal growth factor receptor blockade by antibody IMC-C225 inhibits growth of a human pancreatic carcinoma xenograft in nude mice. Cancer. 2000;89(1):74–82. doi:10.1002/1097-0142(20000701)89:1<74:AID-CNCR11>3.0.CO;2-K.
Watanabe M, Nobuta A, Tanaka J, Asaka M. An effect of K-ras gene mutation on epidermal growth factor receptor signal transduction in PANC-1 pancreatic carcinoma cells. Int J Cancer. 1996;67(2):264–8. doi:10.1002/(SICI)1097-0215(19960717)67:2<264:AID-IJC18>3.0.CO;2-B.
Sibilia M, Fleischmann A, Behrens A, Stingl L, Carroll J, Watt FM, et al. The EGF receptor provides an essential survival signal for SOS-dependent skin tumor development. Cell 2000;102(2):211–20. pii: S0092-8674(00)00026-X.
Day JD, Digiuseppe JA, Yeo C, Lai-Goldman M, Anderson SM, Goodman SN, et al. Immunohistochemical evaluation of HER-2/neu expression in pancreatic adenocarcinoma and pancreatic intraepithelial neoplasms. Hum Pathol. 1996;27(2):119–24.
Marchbank T, Chinery R, Hanby AM, Poulsom R, Elia G, Playford RJ. Distribution and expression of pancreatic secretory trypsin inhibitor and its possible role in epithelial restitution. Am J Pathol. 1996;148(3):715–22.
Bardeesy N, DePinho RA. Pancreatic cancer biology and genetics. Nat Rev Cancer. 2002;2(12):897–909. doi:10.1038/nrc949.
Bockman DE, Guo J, Buchler P, Muller MW, Bergmann F, Friess H. Origin and development of the precursor lesions in experimental pancreatic cancer in rats. Lab Investig. 2003;83(6):853–9.
Jimenez RE, Z’Graggen K, Hartwig W, Graeme-Cook F, Warshaw AL, Fernandez-del Castillo C. Immunohistochemical characterization of pancreatic tumors induced by dimethylbenzanthracene in rats. Am J Pathol. 1999;154(4):1223–9.
Slack JM. Metaplasia and transdifferentiation: from pure biology to the clinic. Nat Rev Mol Cell Biol. 2007;8(5):369–78. doi:10.1038/nrm2146.
Siveke JT, Einwachter H, Sipos B, Lubeseder-Martellato C, Kloppel G, Schmid RM. Concomitant pancreatic activation of Kras(G12D) and Tgfa results in cystic papillary neoplasms reminiscent of human IPMN. Cancer Cell. 2007;12(3):266–79. doi:10.1016/j.ccr.2007.08.002.
Yeh TS, Jan YY, Chiu CT, Ho YB, Chen TC, Lee KF, et al. Characterisation of oestrogen receptor, progesterone receptor, trefoil factor 1, and epidermal growth factor and its receptor in pancreatic cystic neoplasms and pancreatic ductal adenocarcinoma. Gut. 2002;51(5):712–6.
Yeh TS, Tseng JH, Liu NJ, Chen TC, Jan YY, Chen MF. Significance of cellular distribution of ezrin in pancreatic cystic neoplasms and ductal adenocarcinoma. Arch Surg. 2005;140(12):1184–90. doi:10.1001/archsurg.140.12.1184.
Acknowledgments
The authors thank Ms. Yumi Otake and Ms. Michiyo Nakata for excellent technical assistance. This work was supported in part by a KAKENHI (Grant-in-Aid for Scientific Research) in Priority Areas “Integrative Research Toward the Conquest of Cancer” and a Grant-in-Aid for Young Scientists (B) from the Ministry of Education, Culture, Sports, Science and Technology of Japan, a grant from the Osaka Foundation for Promotion of Clinical Immunology, and a grant from Pancreas Research Foundation of Japan.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Ozaki, N., Ohmuraya, M., Ida, S. et al. Serine protease inhibitor Kazal type 1 and epidermal growth factor receptor are expressed in pancreatic tubular adenocarcinoma, intraductal papillary mucinous neoplasm, and pancreatic intraepithelial neoplasia. J Hepatobiliary Pancreat Sci 20, 620–627 (2013). https://doi.org/10.1007/s00534-012-0587-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00534-012-0587-6