Abstract
Purpose
Recommendations for antiemetic prophylaxis supportive to radiotherapy and concomitant chemotherapy are not evidence-based. The purpose of this study was to evaluate the efficacy of the antiemetic regimen concurrent to fractionated radiotherapy and concomitant weekly cisplatin in two Danish departments of oncology.
Methods
Patients with gynecological cancer scheduled to receive fractionated radiotherapy and concomitant weekly cisplatin (40 mg/m2) were asked to complete a study diary in order to assess episodes of emesis, grade of nausea, and use of rescue antiemetic treatment daily during 5 weeks of treatment. Antiemetic treatment consisted of palonosetron and prednisolone. A patient had completed the study if emesis occurred or if 5 weeks of treatment were accomplished without emesis. The primary endpoint was sustained no emesis during 5 weeks of treatment.
Results
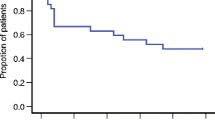
A total of 48 patients completed 155 weekly cycles of radiotherapy, concomitant weekly cisplatin, and antiemetic prophylaxis. The probability of completing 5 cycles without emesis (sustained no emesis) was 57 %. During cycle 1, 42 % of the patients were free from nausea. After 5 cycles, only 23 % of patients were continuously free from nausea. One half of the patients used rescue antiemetic treatment at least once during the 5 cycles.
Conclusion
The present study demonstrates that an antiemetic prophylaxis consisting of palonosetron and prednisolone is insufficient for the prevention of nausea and vomiting induced by radiotherapy and weekly cisplatin in patients treated for gynecological cancer. The addition of a neurokinin-1 receptor antagonist should be investigated in a randomized, double-blind study in this setting.



Similar content being viewed by others
References
Wendt TG, Grabenbauer GG, Rodel CM et al (1998) Simultaneous radiochemotherapy versus radiotherapy alone in advanced head and neck cancer: a randomized multicenter study. J Clin Oncol 16:1318–1324
Keys HM, Bundy BN, Stehman FB et al (1999) Cisplatin, radiation, and adjuvant hysterectomy compared with radiation and adjuvant hysterectomy for bulky stage IB cervical carcinoma. N Engl J Med 340:1154–1161
Stehman FB, Ali S, Keys HM et al (2007) Radiation therapy with or without weekly cisplatin for bulky stage 1B cervical carcinoma: follow-up of a Gynecologic Oncology Group trial. Am J Obstet Gynecol 197:503, e501-506
Adelstein DJ, Li Y, Adams GL et al (2003) An intergroup phase III comparison of standard radiation therapy and two schedules of concurrent chemoradiotherapy in patients with unresectable squamous cell head and neck cancer. J Clin Oncol 21:92–98
Peters WA 3rd, Liu PY, Barrett RJ 2nd et al (2000) Concurrent chemotherapy and pelvic radiation therapy compared with pelvic radiation therapy alone as adjuvant therapy after radical surgery in high-risk early-stage cancer of the cervix. J Clin Oncol 18:1606–1613
Feyer PC, Maranzano E, Molassiotis A et al (2011) Radiotherapy-induced nausea and vomiting (RINV): MASCC/ESMO guideline for antiemetics in radiotherapy: update 2009. Support Care Cancer 19(Suppl 1):S5–14
Roila F, Herrstedt J, Aapro M et al (2010) Guideline update for MASCC and ESMO in the prevention of chemotherapy- and radiotherapy-induced nausea and vomiting: results of the Perugia consensus conference. Ann Oncol 21(Suppl 5):v232–243
Basch E, Prestrud AA, Hesketh PJ et al (2011) Antiemetics: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol 29:4189–4198
Hesketh PJ, Grunberg SM, Gralla RJ et al (2003) The oral neurokinin-1 antagonist aprepitant for the prevention of chemotherapy-induced nausea and vomiting: a multinational, randomized, double-blind, placebo-controlled trial in patients receiving high-dose cisplatin–the Aprepitant Protocol 052 Study Group. J Clin Oncol 21:4112–4119
Ruhlmann C, Herrstedt J (2010) Palonosetron hydrochloride for the prevention of chemotherapy-induced nausea and vomiting. Expert Rev Anticancer Ther 10:137–148
Eisenberg P, Figueroa-Vadillo J, Zamora R et al (2003) Improved prevention of moderately emetogenic chemotherapy-induced nausea and vomiting with palonosetron, a pharmacologically novel 5-HT3 receptor antagonist: results of a phase III, single-dose trial versus dolasetron. Cancer 98:2473–2482
Gralla R, Lichinitser M, Van Der Vegt S et al (2003) Palonosetron improves prevention of chemotherapy-induced nausea and vomiting following moderately emetogenic chemotherapy: results of a double-blind randomized phase III trial comparing single doses of palonosetron with ondansetron. Ann Oncol 14:1570–1577
Saito M, Aogi K, Sekine I et al (2009) Palonosetron plus dexamethasone versus granisetron plus dexamethasone for prevention of nausea and vomiting during chemotherapy: a double-blind, double-dummy, randomised, comparative phase III trial. Lancet Oncol 10:115–124
Del Favero A, Roila F, Basurto C et al (1990) Assessment of nausea. Eur J Clin Pharmacol 38:115–120
Aogi K, Sakai H, Yoshizawa H et al (2012) A phase III open-label study to assess safety and efficacy of palonosetron for preventing chemotherapy-induced nausea and vomiting (CINV) in repeated cycles of emetogenic chemotherapy. Support Care Cancer 20:1507–1514
Lorusso V, Giampaglia M, Petrucelli L et al (2012) Antiemetic efficacy of single-dose palonosetron and dexamethasone in patients receiving multiple cycles of multiple day-based chemotherapy. Support Care Cancer 20:3241–6
de Wit R, van den Berg H, Burghouts J et al (1998) Initial high anti-emetic efficacy of granisetron with dexamethasone is not maintained over repeated cycles. Br J Cancer 77:1487–1491
Herrstedt J, Muss HB, Warr DG et al (2005) Efficacy and tolerability of aprepitant for the prevention of chemotherapy-induced nausea and emesis over multiple cycles of moderately emetogenic chemotherapy. Cancer 104:1548–1555
Franzen L, Nyman J, Hagberg H et al (1996) A randomised placebo controlled study with ondansetron in patients undergoing fractionated radiotherapy. Ann Oncol 7:587–592
Lanciano R, Sherman DM, Michalski J et al (2001) The efficacy and safety of once-daily Kytril (granisetron hydrochloride) tablets in the prophylaxis of nausea and emesis following fractionated upper abdominal radiotherapy. Cancer Investig 19:763–772
Priestman TJ, Roberts JT, Upadhyaya BK (1993) A prospective randomized double-blind trial comparing ondansetron versus prochlorperazine for the prevention of nausea and vomiting in patients undergoing fractionated radiotherapy. Clin Oncol (R Coll Radiol) 5:358–363
Poli-Bigelli S, Rodrigues-Pereira J, Carides AD et al (2003) Addition of the neurokinin 1 receptor antagonist aprepitant to standard antiemetic therapy improves control of chemotherapy-induced nausea and vomiting. Results from a randomized, double-blind, placebo-controlled trial in Latin America. Cancer 97:3090–3098
Albany C, Brames MJ, Fausel C et al (2012) Randomized, double-blind, placebo-controlled, phase III cross-over study evaluating the oral neurokinin-1 antagonist aprepitant in combination with a 5HT3 receptor antagonist and dexamethasone in patients with germ cell tumors receiving 5-day cisplatin combination chemotherapy regimens: a hoosier oncology group study. J Clin Oncol 30:3998–4003
Einhorn LH, Brames MJ, Dreicer R et al (2007) Palonosetron plus dexamethasone for prevention of chemotherapy-induced nausea and vomiting in patients receiving multiple-day cisplatin chemotherapy for germ cell cancer. Support Care Cancer 15:1293–1300
Herrstedt J, Sigsgaard TC, Nielsen HA et al (2007) Randomized, double-blind trial comparing the antiemetic effect of tropisetron plus metopimazine with tropisetron plus placebo in patients receiving multiple cycles of multiple-day cisplatin-based chemotherapy. Support Care Cancer 15:417–426
Enblom A, Bergius Axelsson B, Steineck G et al (2009) One third of patients with radiotherapy-induced nausea consider their antiemetic treatment insufficient. Support Care Cancer 17:23–32
Maranzano E, De Angelis V, Pergolizzi S et al (2010) A prospective observational trial on emesis in radiotherapy: analysis of 1020 patients recruited in 45 Italian radiation oncology centres. Radiother Oncol 94:36–41
Conflict of interest
The authors declare no conflicts of interest in this work. The authors have full control of all primary data and data is available for the reviewers upon request. The study was investigator-initiated and no funding was received.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ruhlmann, C.H., Belli, C., Dahl, T. et al. Palonosetron and prednisolone for the prevention of nausea and emesis during fractionated radiotherapy and 5 cycles of concomitant weekly cisplatin—a phase II study. Support Care Cancer 21, 3425–3431 (2013). https://doi.org/10.1007/s00520-013-1926-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-013-1926-0