Abstract
Key message
Sustainable stomatal opening despite xylem cavitation occurs in ring-porous species and stomatal closure prior to cavitation in diffuse-porous species during soil drought.
Abstract
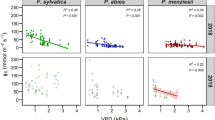
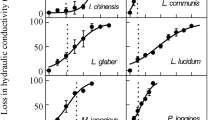
To elucidate the relationship between water loss regulation and vulnerability to cavitation associated with xylem structure, stomatal conductance (g s), defoliation, vulnerability curves, and vessel features were measured on seedlings of ring-porous Zelkova serrata and Melia azedarach, and diffuse-porous Betula platyphylla var. japonica, Cerasus jamasakura and Carpinus tschonoskii. Under prolonged drought conditions, the percentage loss of hydraulic conductivity (PLC) increased and g s decreased gradually with decreasing predawn (Ψpd) or xylem water potential (Ψxylem) in Z. serrata. During the gentle increase of PLC in M. azedarach, g s increased in the early stages of dehydration while leaves were partly shed. A sharp reduction in g s was observed before the onset of an increase in the PLC for drying plants of the three diffuse-porous species, suggesting cavitation avoidance by stomatal regulation. In the ring-porous species, xylem-specific hydraulic conductivity (K s) was higher, whereas the vessel multiple fractions, the ratio of the number of grouped vessels to total vessels, was lower than that in the diffuse-porous species, suggesting that many were distributed as solitary vessels. This may explain the gradual increase in the PLC with decreasing Ψxylem because isolated vessels provide less opportunity for air seeding. Different water loss regulation to soil drought was identified among the species, with potential mechanisms being sustainable gas exchange at the expense of xylem dysfunction or partial leaf shedding, and the avoidance of xylem cavitation by strict stomatal regulation. These were linked to vulnerability to cavitation that appears to be governed by xylem structural properties.






Similar content being viewed by others
References
Blackman CJ, Brodribb TJ, Jordan GJ (2009) Leaf hydraulics and drought stress: response, recovery and survivorship in four woody temperate plant species. Plant Cell Environ 32:1584–1595
Boura A, Franceschi DD (2007) Is porous wood structure exclusive of deciduous trees? C R Palevol 6:385–391
Bréda N, Huc R, Granier A, Dreyer E (2006) Temperate forest trees and stands under severe drought: a review of ecophysiological responses, adaptation processes and long-term consequences. Ann For Sci 63:625–644
Brodribb TJ, Holbrook NM, Edwards EJ, Gutiérrez MV (2003) Relations between stomatal closure, leaf turgor and xylem vulnerability in eight tropical dry forest trees. Plant Cell Environ 26:443–450
Buckley TN (2005) The control of stomata by water balance. New Phytol 168:275–292
Bush SE, Pataki DE, Hultine KR, West AG, Sperry JS, Ehleringer JR (2008) Wood anatomy constrains stomatal responses to atmospheric vapor pressure deficit in irrigated, urban trees. Oecol 156:13–20
Christman MA, Sperry JS, Smith DD (2011) Rare pits, large vessels and extreme vulnerability to cavitation in a ring-porous tree species. New Phytol 193:713–720
Cochard H, Tyree MT (1990) Xylem dysfunction in Quercus: vessel size, tyloses, cavitation and seasonal changes in embolism. Tree Physiol 6:393–407
Cochard H, Herbette T, Barigah T, Badel E, Ennajeh M, Vilagrosa A (2010) Does sample length influence the shape of xylem embolism vulnerability curves? A test with the Cavitron spinning technique. Plant Cell Environ 33:1543–1552
Davis SD, Ewers FW, Sperry JS, Portwood KA, Crocker MC, Adams GC (2002) Shoot dieback during prolonged drought in Ceanothus (Rhamnaceae) chaparral of California: a possible case of hydraulic failure. Am J Bot 89:820–828
Easterling DR, Meehl GA, Parmesan C, Changnon SA, Karl TR, Mearns LO (2000) Climate extremes: observations, modeling, and impacts. Sci 289:2068–2074
Ellmore GS, Zanne AE, Orians CM (2006) Comparative sectoriality in temperate hardwoods: hydraulics and xylem anatomy. Bot J Linn Soc 150:61–71
Hacke UG, Sperry JS, Pockman WT, Davis SD, McCulloh MA (2001) Trends in wood density and structure are linked to prevention of xylem implosion by negative pressure. Oecol 126:457–461
Hacke UG, Sperry JS, Wheeler JK, Castro L (2006) Scaling of angiosperm xylem structure with safety and efficiency. Tree Physiol 26:689–701
Hölscher D, Koch O, Korn S, Leuschner C (2005) Sap flux of five co-occurring tree species in a temperate broad-leaved forest during seasonal soil drought. Trees 19:628–637
IAWA Committee (1989) IAWA list of microscopic features for hardwood identification. IAWA Bull n.s. 10:219–332
IPCC (2007) Climate Change 2007: the physical science basis. Contribution of working group I to the fourth assessment report of the intergovernmental panel on climate change. http://www.ipcc.ch/pdf/assessment-report/ar4/wg1/ar4_wg1_full_report.pdf. Accessed 26 Aug 2013
Iwashita T, Yamamoto R (1993) A statistical analysis of the extreme events: long-term trend of heavy daily precipitation. J Meteorol Soc Jpn 71:637–640
Jacobsen AL, Ewers FW, Pratt RB, Paddock WA III, Davis SD (2005) Do xylem fibers affect vessel cavitation resistance? Plant Physiol 139:546–556
Jacobsen AL, Pratt RB, Ewers FW, Davis SD (2007) Cavitation resistance among 26 chaparral species of Southern California. Ecol Monogr 77:99–115
Jansen S, Choat B, Pletsers A (2009) Morphological variation of intervessel pit membranes and implications to xylem function in angiosperms. Am J Bot 96:409–419
Jones HG, Sutherland RA (1991) Stomatal control of xylem embolism. Plant Cell Environ 14:607–612
Kosugi Y, Takanashi S, Tani M, Ohkubo S, Matsuo N, Itoh M, Noguchi S, Nik AR (2011) Effect of inter-annual climate variability on evapotranspiration and canopy CO2 exchange of a tropical rainforest in Peninsular Malaysia. J For Res 17:227–240
McDowell N, Pockman WT, Allen CD, Breshears DD, Cobb N, Kolb T, Plaut J, Sperry J, West A, Williams DG, Yepez EA (2008) Mechanisms of plant survival and mortality during drought: why do some plants survive while others succumb to drought? New Phytol 178:719–739
Meinzer FC, Grants DA (1990) Stomatal and hydraulic conductance in growing sugarcane: stomatal adjustment to water transport capacity. Plant Cell Environ 13:383–388
Meinzer FC, Johnson DM, Lachenbruch B, McCulloh KA, Woodruff DR (2009) Xylem hydraulic safety margins in woody plants: coordination of stomatal control of xylem tension with hydraulic capacitance. Funct Ecol 23:922–930
Ogasa M, Miki N, Yoshikawa K (2010) Changes of hydraulic conductivity during dehydration and rehydration in Quercus serrata Thunb. and Betula platyphylla var. japonica Hara: the effect of xylem structures. Tree Physiol 30:608–617
Ogasa M, Miki HN, Murakami Y, Yoshikawa K (2013) Recovery performance in xylem hydraulic conductivity is correlated with cavitation resistance for temperate deciduous tree species. Tree Physiol 33:335–344
Oren R, Pataki DE (2001) Transpiration in response to variation in microclimate and soil moisture in southeastern deciduous forests. Oecol 127:549–559
Peters EB, McFadden JP, Montgomery RA (2010) Biological and environmental controls on tree transpiration in a suburban landscape. J Geophys Res 115:G04006
Saliendra NZ, Sperry JS, Comstock JP (1995) Influence of leaf water status on stomatal response to humidity, hydraulic conductance, and soil drought in Betula occidentalis. Planta 196:357–366
Salleo S, Nardini A, Pitt F, LoGullo MA (2000) Xylem cavitation and hydraulic control of stomatal conductance in Laurel (Laurus nobilis L.). Plant Cell Environ 23:71–79
Sano Y, Morris H, Shimada H, De Craene LPR, Jansen S (2011) Anatomical features associated with water transport in imperforate trancheary elements of vessel-bearing angiosperms. Ann Bot 107:953–964
Sperry JS, Tyree MT (1988) Mechanism of water stress-induced xylem embolism. Plant Physiol 88:581–587
Sperry JS, Donnelly JR, Tyree MT (1988) A method for measuring hydraulic conductivity and embolism in xylem. Plant Cell Environ 11:35–40
Sperry JS, Nichols KL, Sullivan JEM, Eastlack SE (1994) Xylem embolism in ring-porous, diffuse-porous, and coniferous trees of Northern Utah and Interior Alaska. Ecol 75:1736–1752
Taneda H, Sperry JS (2008) A case-study of water transport in co-occurring ring- versus diffuse-porous trees: contrasts in water-status, conducting capacity, cavitation and vessel refilling. Tree Physiol 28:1641–1651
Tyree MT, Sperry JS (1988) Do woody plants operate near the point of catastrophic xylem dysfunction caused by dynamic water stress? Plant Physiol 88:574–580
Tyree MT, Zimmermann MH (2002) Xylem structure and the ascent of sap, 2nd edn. Springer, Berlin
Tyree M, Cochard H, Cruiziat P, Sinclair B, Améglio T (1993) Drought-induced leaf shedding in walnut: evidence for vulnerability segmentation. Plant Cell Environ 16:879–882
Vilagrosa A, Bellot J, Vallejo VR, Gil-Pelegrin E (2003) Cavitation, stomatal conductance, and leaf dieback in seedlings of two co-occurring Mediterranean shrubs during an intense drought. J Exp Bot 54:2015–2024
Wheeler JK, Sperry JS, Hacke UG, Hoang N (2005) Inter-vessel pitting and cavitation in woody Rosaceae and other vesselled plants: a basis for a safety versus efficiency trade-off in xylem transport. Plant Cell Environ 28:800–812
Acknowledgments
We thank Dr. Keiji Sakamoto and Dr. Muneto Hirobe for their helpful comments. We also thank Dr. Shogo Imada, Dr. Fumiko Iwanaga, the staff of the Arid Land Research Center, Tottori University, and Yuki Murakami, Yuma Yamaguchi, Ichido Yoshimori, Yasuaki Akaji, Marina Itohara, Akiko Kobayashi, Satoshi Sasaki, Shoko Masaki, Yasuko Yoshioka, and Tomo Aihara for their technical and sustained support during the experiments.
This work was funded by a Grant-in-Aid for Japan Society for the Promotion of Science (JSPS) Fellows (21.5030) to M.O. and was conducted under the Cooperative Research Program of the Arid Land Research Center, Tottori University.
Conflict of interest
None declared.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by M. Zwieniecki.
Rights and permissions
About this article
Cite this article
Ogasa, M., Miki, N.H., Okamoto, M. et al. Water loss regulation to soil drought associated with xylem vulnerability to cavitation in temperate ring-porous and diffuse-porous tree seedlings. Trees 28, 461–469 (2014). https://doi.org/10.1007/s00468-013-0963-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00468-013-0963-0