Abstract
Background
Prevention of chronic kidney allograft injury (CAI) is a major goal in improving kidney allograft survival; however, the mechanisms of CAI are not clearly understood. The current study investigated whether alternatively activated M2-type macrophages are involved in the development of CAI.
Methods
A retrospective study examined kidney allograft protocol biopsies (at 1 h and at years 1, 5, and 10—a total of 41 biopsies) obtained from 13 children undergoing transplantation between 1991 and 2008 who were diagnosed with CAI: interstitial fibrosis and tubular atrophy (IF/TA) not otherwise specified (IF/TA-NOS).
Results
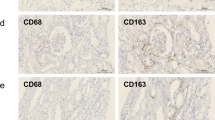
Immunostaining identified a significant increase in interstitial fibrosis with accumulation of CD68 + CD163+ M2-type macrophages. CD163+ cells were frequently localized to areas of interstitial fibrosis exhibiting collagen I deposition and accumulation of α-smooth muscle actin (SMA) + myofibroblasts. There was a significant correlation between interstitial CD163+ cells and the parameters of interstitial fibrosis (p < 0.0001), and kidney function (r =−0.82, p < 0.0001). The number of interstitial CD163+ cells at years 1 and 5 also correlated with parameters of interstitial fibrosis at years 5 and 10 respectively. Notably, urine CD163 levels correlated with interstitial CD163+ cells (r = 0.79, p < 0.01) and parameters of interstitial fibrosis (p < 0.0001). However, CD3+ T lymphocytic infiltration did not correlate with macrophage accumulation or fibrosis. In vitro, dexamethasone up-regulated expression of CD163 and cytokines (TGF-β1, FGF-2, CTGF) in human monocyte-derived macrophages, indicating a pro-fibrotic phenotype.
Conclusions
Our findings identify a major population of M2-type macrophages in patients with CAI, and suggest that these M2-type macrophages might promote the development of interstitial fibrosis in IF/TA-NOS.






Similar content being viewed by others
References
Solez K, Axelsen RA, Benediktsson H, Burdick JF, Cohen AH, Colvin RB, Croker BP, Droz D, Dunnill MS, Halloran PF (1993) International standardization of criteria for the histologic diagnosis of renal allograft rejection: the Banff working classification of kidney transplant pathology. Kidney Int 44:411–422
Shrestha BM, Haylor J (2014) Biological pathways and potential targets for prevention and therapy of chronic allograft nephropathy. Biomed Res Int 2014:482438
Solez K, Colvin RB, Racusen LC, Sis B, Halloran PF, Birk PE, Campbell PM, Cascalho M, Collins AB, Demetris AJ, Drachenberg CB, Gibson IW, Grimm PC, Haas M, Lerut E, Liapis H, Mannon RB, Marcus PB, Mengel M, Mihatsch MJ, Nankivell BJ, Nickeleit V, Papadimitriou JC, Platt JL, Randhawa P, Roberts I, Salinas-Madriga L, Salomon DR, Seron D, Sheaff M, Weening JJ (2007) Banff ‘05 Meeting Report: differential diagnosis of chronic allograft injury and elimination of chronic allograft nephropathy (‘CAN’). Am J Transplant 7:518–526
Solez K, Colvin RB, Racusen LC, Haas M, Sis B, Mengel M, Halloran PF, Baldwin W, Banfi G, Collins AB, Cosio F, David DS, Drachenberg C, Einecke G, Fogo AB, Gibson IW, Glotz D, Iskandar SS, Kraus E, Lerut E, Mannon RB, Mihatsch M, Nankivell BJ, Nickeleit V, Papadimitriou JC, Randhawa P, Regele H, Renaudin K, Roberts I, Seron D, Smith RN, Valente M (2008) Banff 07 classification of renal allograft pathology: updates and future directions. Am J Transplant 8:753–760
Croker BP, Clapp WL, Abu Shamat AR, Kone BC, Peterson JC (1996) Macrophages and chronic renal allograft nephropathy. Kidney Int Suppl 57:S42–S49
Liu G, Yang H (2013) Modulation of macrophage activation and programming in immunity. J Cell Physiol 228:502–512
Mantovani A, Biswas SK, Galdiero MR, Sica A, Locati M (2013) Macrophage plasticity and polarization in tissue repair and remodelling. J Pathol 229:176–186
Anders HJ, Ryu M (2011) Renal microenvironments and macrophage phenotypes determine progression or resolution of renal inflammation and fibrosis. Kidney Int 80:915–925
Mannon RB (2012) Macrophages: contributors to allograft dysfunction, repair, or innocent bystanders? Curr Opin Organ Transplant 17:20–25
Ikezumi Y, Suzuki T, Karasawa T, Hasegawa H, Yamada T, Imai N, Narita I, Kawachi H, Polkinghorne KR, Nikolic-Paterson DJ, Uchiyama M (2011) Identification of alternatively activated macrophages in new-onset paediatric and adult immunoglobulin A nephropathy: potential role in mesangial matrix expansion. Histopathology 58:198–210
Ikezumi Y, Suzuki T, Karasawa T, Hasegawa H, Kawachi H, Nikolic-Paterson DJ, Uchiyama M (2010) Contrasting effects of steroids and mizoribine on macrophage activation and glomerular lesions in rat thy-1 mesangial proliferative glomerulonephritis. Am J Nephrol 31:273–282
Lan HY, Mu W, Nikolic-Paterson DJ, Atkins RC (1995) A novel, simple, reliable and sensitive method for multiple immunoenzymic staining: use of microwave oven heating to block antibody crossreactivity and retrieve antigens. J Histochem Cytochem 43:97–102
Han Y, Ma FY, Tesch GH, Manthey CL, Nikolic-Paterson DJ (2013) Role of macrophages in the fibrotic phase of rat crescentic glomerulonephritis. Am J Physiol Renal Physiol 304:F1043–1053
Tse GH, Hughes J (2013) Macrophages and transplant rejection: a novel future target. Transplantation 96:946–948
Kanlaya R, Sintiprungrat K, Thongboonkerd V (2013) Secreted products of macrophages exposed to calcium oxalate crystals induce epithelial mesenchymal transition of renal tubular cells via RhoA-dependent TGF-β1 pathway. Cell Biochem Biophys 67:1207–1215
Tan TK, Zheng G, Hsu TT, Lee SR, Zhang J, Zhao Y, Tian X, Wang Y, Wang YM, Cao Q, Wang Y, Lee VW, Wang C, Zheng D, Alexander SI, Thompson E, Harris DC (2013) Matrix metalloproteinase-9 of tubular and macrophage origin contributes to the pathogenesis of renal fibrosis via macrophage recruitment through osteopontin cleavage. Lab Invest 93:434–449
Ko GJ, Boo CS, Jo SK, Cho WY, Kim HK (2008) Macrophages contribute to the development of renal fibrosis following ischaemia/reperfusion-induced acute kidney injury. Nephrol Dial Transplant 23:842–852
Alidousty C, Rauen T, Hanssen L, Wang Q, Alampour-Rajabi S, Mertens PR, Bernhagen J, Floege J, Ostendorf T, Raffetseder U (2014) Calcineurin-mediated YB-1 dephosphorylation regulates CCL5 expression during monocyte differentiation. J Biol Chem 289:21401–21412
Höcker B, Tönshoff B (2009) Treatment strategies to minimize or prevent chronic allograft dysfunction in pediatric renal transplant recipients: an overview. Paediatr Drugs 11:381–396
Yang H (2006) Maintenance immunosuppression regimens: conversion, minimization, withdrawal, and avoidance. Am J Kidney Dis 47:S37–51
Ishikawa H (1999) Mizoribine and mycophenolate mofetil. Curr Med Chem 6:575–597
Ju MK, Huh KH, Park KT, Kim SJ, Cho BH, Kim CD, So BJ, Kang CM, Lee S, Joo DJ, Kim YS (2013) Mizoribine versus mycophenolate mofetil in combination therapy with tacrolimus for de novo kidney transplantation: evaluation of efficacy and safety. Transplant Proc 45:1481–1486
Helal I, Chan L (2011) Steroid and calcineurin inhibitor-sparing protocols in kidney transplantation. Transplant Proc 43:472–477
Kristiansen M, Graversen JH, Jacobsen C, Sonne O, Hoffman HJ, Law SK, Moestrup SK (2001) Identification of the haemoglobin scavenger receptor. Nature 409(6817):198–201
Acknowledgements
This work was supported by Grants-in-Aid for Scientific Research (25461618 to Y.I.) from the Ministry of Education, Culture, Sports, Science and Technology of Japan, and Grants-in-Aid for Promotion of Niigata University Research Projects from Niigata University.
Conflicts of interest
No conflicts of interest to declare.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ikezumi, Y., Suzuki, T., Yamada, T. et al. Alternatively activated macrophages in the pathogenesis of chronic kidney allograft injury. Pediatr Nephrol 30, 1007–1017 (2015). https://doi.org/10.1007/s00467-014-3023-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-014-3023-0