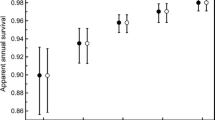
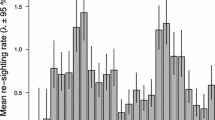
Abstract
In many species, temporary emigration (TE) is a phenomenon, often indicative of life-history characteristics such as dormancy, skipped reproduction, or partial migration, whereby certain individuals in a population are temporarily unobservable at a particular site. TE may be a flexible condition-dependent strategy that allows individuals to mitigate effects of adverse conditions. Consequently, TE rates ought to be highly variable, but sources of variations are poorly understood for most species. We used data from known-aged female Weddell seals (Leptonychotes weddellii) tagged in Erebus Bay, Antarctica, to investigate sources of variation in TE rates prior to reproduction and to evaluate possible implications for age-specific probability of first reproduction. TE rates were near 1 the year after birth, decreased to an average of 0.15 (\( \widehat{\text{SE}} \) = 0.01) by age 8, and were similar thereafter. TE rates varied substantially from year-to-year and were lower for seals that attended reproductive colonies the previous year than for seals that did not attend (e.g., \( \overline{{\hat{\psi }_{{i,{\text{age}}\,8}}^{\text{UU}} - \hat{\psi }_{{i,\,{\text{age}}\,8}}^{\text{PU}} }} \) = 0.22). Recruitment rates were marginally greater for seals that did attend than for seals that did not attend colonies the previous year. For Weddell seals specifically, our results suggest that (1) motivation to attend colonies varied temporally, (2) as seals grew older they had increased motivation to attend even before reproductive maturity, and (3) seals appear to follow various attendance strategies. More broadly, our results support the idea of TE as a variable, condition-dependent strategy, and highlight the utility of TE models for providing population and life-history insights for diverse taxa.




Similar content being viewed by others
References
Ainley DG (2002) The Ross Sea, Antarctica, where all ecosystem processes still remain for study, but maybe not for long. Mar Ornithol 30:55–62
Arrigo KR, van Dijken GL (2003) Impact of iceberg C-19 on Ross Sea primary production. Geophys Res Lett. doi:10.1029/2003GL017721
Arrigo KR, van Dijken GL, Ainley DG, Fahnestock MA, Markus T (2002) Ecological impact of a large Antarctic iceberg. Geophys Res Lett. doi:10.1029/2001GL014160
Aubry LM, Koons DN, Monnat J-Y, Cam E (2009) Consequences of recruitment decisions and heterogeneity on age-specific breeding success in a long-lived seabird. Ecology 90:2491–2502. doi:10.1890/08-1475.1
Bailey LL, Kendall WL, Church DR, Wilbur HM (2004) Estimating survival and breeding probability for pond-breeding amphibians: a modified robust design. Ecology 85:2456–2466. doi:10.1890/03-0539
Bailey LL, Converse SJ, Kendall WL (2010) Bias, precision, and parameter redundancy in complex multistate models with unobservable states. Ecology 91:1598–1604. doi:10.1890/09-1633.1
Baker JD, Thompson PM (2007) Temporal and spatial variation in age-specific survival rates of a long-lived mammal, the Hawaiian monk seal. Proc R Soc Lond B 274:407–415. doi:10.1098/rspb.2006.3737
Beauplet G, Barbraud C, Chambellant M, Guinet C (2005) Interannual variation in the post-weaning and juvenile survival of subantarctic fur seals: influence of pup sex, growth rate and oceanographic conditions. J Anim Ecol 74:1160–1172. doi:10.1111/j.1365-2656.2005.01016.x
Beauplet G, Barbraud C, Dabin W, Küssener C, Guinet C (2006) Age-specific survival and reproductive performances in fur seals: evidence of senescence and individual quality. Oikos 112:430–441. doi:10.1111/j.0030-1299.2006.14412.x
Bjorndal KA, Bolten AB, Dellinger T, Delgado C, Martins HR (2003) Compensatory growth in oceanic loggerhead sea turtles: response to a stochastic environment. Ecology 84:1237–1249. doi:10.1890/0012-9658(2003)084[1237:CGIOLS]2.0.CO;2
Boyle WA (2008) Partial migration in birds: tests of three hypotheses in a tropical lekking frugivore. J Anim Ecol 77:1122–1128. doi:10.1111/j.1365-2656.2008.01451.x
Bradford AL, Wade PR, Weller DW, Burdin AM, Ivashchenko YV, Tsidulko GA, VanBlaricom GR, Brownell RLB Jr (2006) Survival estimates of western gray whales Eschrichtius robustus incorporating individual heterogeneity and temporary emigration. Mar Ecol Prog Ser 315:293–307. doi:10.3354/meps315293
Brierley AS, Fernandes PG, Brandon MA, Armstrong F, Millard NW, McPhail SD, Stevenson P, Pebody M, Perrett J, Squires M, Bone DG, Griffiths G (2002) Antarctic krill under sea ice: elevated abundance in a narrow band just south of ice edge. Science 295:1890–1892. doi:10.1126/science.1068574
Brodersen J, Nilsson PA, Hansson L-A, Skov C, Brönmark C (2008) Condition-dependent individual decision-making determines cyprinid partial migration. Ecology 89:1195–1200. doi:10.1890/07-1318.1
Burnham KP, Anderson DR (2002) Model Selection and Multimodel Inference; A Practical Information-theoretic Approach, 2nd edn. Springer, New York
Cam E, Monnat J-Y, Hines JE (2003) Long-term fitness consequences of early conditions in the kittiwake. J Anim Ecol 72:411–424. doi:10.1046/j.1365-2656.2003.00708.x
Cameron MF, Siniff DB (2004) Age-specific survival, abundance, and immigration rates of a Weddell seal (Leptonychotes weddellii) population in McMurdo Sound, Antarctica. Can J Zool 82:601–615. doi:10.1139/z04-025
Cameron MF, Siniff DB, Proffitt KM, Garrott RA (2007) Site fidelity of Weddell seals: the effects of sex and age. Antarct Sci 19:149–155. doi:10.1017/S0954102007000223
Conn PB, Cooch EG (2009) Multistate capture–recapture analysis under imperfect state observation: an application to disease models. J Appl Ecol 46:486–492. doi:10.1111/j.1365-2664.2008.01597.x
Converse SJ, Kendall WL, Doherty PF, Ryan PG (2009) Multistate models for estimation of survival and reproduction in the grey-headed albatross (Thalassarche chrysostoma). Auk 126:77–88. doi:10.1525/auk.2009.07189
Crespin L, Harris MP, Lebreton J-D, Frederiksen M, Wanless S (2006) Recruitment to a seabird population depends on environmental factors and on population size. J Anim Ecol 75:228–238. doi:10.1111/j.1365-2656.2006.01035.x
Danchin E, Giraldeau L-A, Valone TJ, Wagner RH (2004) Public information: from nosy neighbors to cultural evolution. Science 305:487–491. doi:10.1126/science.1098254
de Bruyn PJN, Tosh CA, Bester MN, Cameron EZ, McIntyre T, Wilkinson IS (2011) Sex at sea: alternative mating system in an extremely polygynous mammal. Anim Behav 82:445–451. doi:10.1016/j.anbehav.2011.06.006
Deutsch JC, Nefdt RJC (1992) Olfactory cues influence female choice in two lek-breeding antelopes. Nature 356:596–598. doi:10.1038/356596a0
Dingle H (1996) Migration: The Biology of Life on the Move. Oxford University Press, New York
Dobson FS, Jones WT (1985) Multiple causes of dispersal. Am Nat 126:855–858
Doherty PF, White GC, Burnham KP (2010) Comparison of model building and selection strategies. J Ornithol. doi:10.1007/s10336-010-0598-5
Doligez B, Danchin E, Clobert J (2002) Public information and breeding habitat selection in a wild bird population. Science 297:1168–1170. doi:10.1126/science.1072838
Dugger KM, Ainley DG, Lyver PO, Barton K, Ballard G (2010) Survival differences and the effect of environmental instability on breeding dispersal in an Adélie penguin meta-population. Proc Natl Acad Sci USA 107:12375–12380. doi:10.1073/pnas.1000623107
Eisert R, Oftedal OT, Lever M, Ramdohr S, Breier BH, Barrell GK (2005) Detection of food intake in a marine mammal using marine osmolytes and their analogues as dietary biomarkers. Mar Ecol Prog Ser 300:213–228. doi:10.3354/meps300213
Forcada J, Trathan PN, Murphy EJ (2008) Life history buffering in Antarctic mammals and birds against changing patterns of climate and environmental variation. Glob Change Biol 14:2473–2488. doi:10.1111/j.1365-2486.2008.01678.x
Forchhammer MC, Clutton-Brock TH, Lindström J, Albon SD (2001) Climate and population density induce long-term cohort variation in a northern ungulate. J Anim Ecol 70:721–729. doi:10.1046/j.0021-8790.2001.00532.x
Frederiksen M, Bregnballe T (2000) Diagnosing a decline in return rate of 1-year-old cormorants: mortality, emigration or delayed return? J Anim Ecol 69:753–761. doi:10.1046/j.1365-2656.2000.00436.x
Fujiwara M, Caswell H (2002) A general approach to temporary emigration in mark-recapture analysis. Ecology 83:3266–3275. doi:10.1890/0012-9658(2002)083[3266:AGATTE]2.0.CO;2
Gaillard J-M, Boutin J-M, Delorme D, Van Laere G, Duncan P, Lebreton J-D (1997) Early survival in roe deer: causes and consequences of cohort variation in two contrasted populations. Oecologia 112:502–513
Garrott RA, Rotella JJ, Siniff DB, Parkinson CL, Stauffer GE (2012) Environmental variation and cohort effects in an Antarctic predator. Oikos 121:1027–1040. doi:10.1111/j.1600-0706.2011.19673.x
Gelatt TS, Davis CS, Siniff DB, Strobeck C (2001) Molecular evidence for twinning in Weddell seals (Leptonychotes weddellii). J Mammal 82:491–499. doi:10.1644/1545-1542(2001)082<0491:MEFTIW>2.0.CO;2
Hadley GL, Rotella JJ, Garrott RA, Nichols JD (2006) Variation in probability of first reproduction of Weddell seals. J Anim Ecol 75:1058–1070. doi:10.1111/j.1365-2656.2006.01118.x
Hadley GL, Rotella JJ, Garrott RA (2007a) Influence of maternal characteristics and oceanographic conditions on survival and recruitment probabilities of Weddell seals. Oikos 116:601–613. doi:10.1111/j.0030-1299.2007.15528.x
Hadley GL, Rotella JJ, Garrott RA (2007b) Evaluation of reproductive costs for Weddell seals in Erebus Bay, Antarctica. J Anim Ecol 76:448–458. doi:10.1111/j.1365-2656.2007.01219.x
Hindell MA (1991) Some life-history parameters of a declining population of southern elephant seals, Mirounga leonina. J Anim Ecol 60:119–134. doi:10.2307/5449
Holyoak M, Casagrandi R, Nathan R, Revilla E, Spiegel O (2008) Trends and missing parts in the study of movement ecology. Proc Natl Acad Sci USA 105:19060–19065. doi:10.1073/pnas.0800483
Hoover JP (2003) Decision rules for site fidelity in a migratory bird, the prothonotary warbler. Ecology 84:416–430. doi:10.1890/0012-9658(2003)084[0416:DRFSFI]2.0.CO;2
Ims RA, Hjermann DØ (2001) Condition-dependent dispersal. In: Clobert J, Danchin E, Dhondt AA, Nichols JD (eds) Dispersal. Oxford University Press, New York, pp 452–465
Jahn AE, Levey DJ, Hostetler JA, Mamani AM (2010) Determinants of partial bird migration in the Amazon Basin. J Anim Ecol 79:983–992. doi:10.1111/j.1365-2656.2010.01713.x
Kendall WL (2004) Coping with unobservable and mis-classified states in capture-recapture studies. Anim Biodivers Cons 27:97–107
Kendall WL (2006) The Robust Design. In: Cooch EG, White GC (eds) Program MARK: A Gentle Introduction, 9th edn. http://www.phidot.org/software/mark/docs/book/
Kendall WL, Bjorkland R (2001) Using open robust design models to estimate temporary emigration from capture-recapture data. Biometrics 57:1113–1122. doi:10.1111/j.0006-341X.2001.01113.x
Kendall WL, Nichols JD (2002) Estimating state-transition probabilities for unobservable states using capture-recapture/resighting data. Ecology 83:3276–3284. doi:10.1890/0012-9658(2002)083[3276:ESTPFU]2.0.CO;2
Kendall WL, Nichols JD, Hines JE (1997) Estimating temporary emigration using capture–recapture data with Pollock’s robust design. Ecology 78:563–578. doi:10.1890/0012-9658(1997)078[0563:ETEUCR]2.0.CO;2
Kéry M, Gregg KB, Schaub M (2005) Demographic estimation methods for plants with unobservable life-states. Oikos 108:307–320. doi:10.1111/j.0030-1299.2005.13589.x
Kooyman GL, Ainley DG, Ballard G, Ponganis PJ (2007) Effects of giant icebergs on two emperor penguin colonies in the Ross Sea, Antarctica. Antarct Sci 19:31–38. doi:10.1017/S0954102007000065
Laake JL (2010) RMark: R Code for MARK Analysis. R package version 1.9.6
Lake SE, Burton HR, Barker RJ, Hindell MA (2008) Annual reproductive rates of Weddell seals in eastern Antarctica from 1973 to 2000. Mar Ecol Prog Ser 366:259–270. doi:10.3354/meps07502
Langtimm CA (2009) Non-random temporary emigration and the robust design: conditions for bias at the end of a time series. Environ Ecol Stat 3:745–761. doi:10.1007/978-0-387-78151-8_34
Langvatn R, Mysterud A, Stenseth NC, Yoccoz NG (2004) Timing and synchrony of ovulation in red deer constrained by short northern summers. Am Nat 163:763–772. doi:10.1086/383594
Laws RM (1956) Growth and sexual maturity in aquatic mammals. Nature 178:193–194. doi:10.1038/178193a0
Lebreton JD, Burnham KP, Clobert J, Anderson DR (1992) Modeling survival and testing biological hypotheses using marked animals—a unified approach with case-studies. Ecol Monogr 62:67–118
Lebreton J-D, Nichols JD, Barker RJ, Pradel R, Spendelow JA (2009) Modeling individual animal histories with multistate capture-recapture models. Adv Ecol Res 41:87–173. doi:10.1016/S0065-2504(09)00403-6
Lescroël A, Ballard G, Toniolo V, Barton KJ, Wilson PR, Lyver POB, Ainley DG (2010) Working less to gain more: when breeding quality relates to foraging efficiency. Ecology 91:2044–2055. doi:10.1890/09-0766.1
Lindström J (1999) Early development and fitness in birds and mammals. Trends Ecol Evol 14:343–348. doi:10.1016/S0169-5347(99)01639-0
Lunn NJ, Boyd IL, Croxall JP (1994) Reproductive performance of female Antarctic fur seals: the influence of age, breeding experience, environmental variation and individual quality. J Anim Ecol 63:827–840
McClintock MK (1978) Estrous synchrony and its mediation by airborne chemical communication (Rattus norvegicus). Horm Behav 10:264–276. doi:10.1016/0018-506X(78)90071-5
McMahon TE, Matter WJ (2006) Linking habitat selection, emigration and population dynamics of freshwater fishes: a synthesis of ideas and approaches. Ecol Freshw Fish 15:200–210. doi:10.1111/j.1600-0633.2006.00130.x
McMahon CR, Burton HR, Bester MN (2003) A demographic comparison of two southern elephant seal populations. J Anim Ecol 72:61–74. doi:10.1046/j.1365-2656.2003.00685.x
Muths E, Scherer RD, Corn PS, Lambert BA (2006) Estimation of temporary emigration in male toads. Ecology 87:1048–1056. doi:10.1890/0012-9658(2006)87[1048:EOTEIM]2.0.CO;2
Nevoux M, Barbraud C (2005) Relationships between sea ice concentration, sea surface temperature and demographic traits of thin-billed prions. Polar Biol 29:445–453. doi:10.1007/s00300-005-0075-4
Nevoux M, Barbraud C (2006) Do demographic responses to climate change depend on life history strategies? J Ornithol 147:25
Nichols JD, Kaiser A (1999) Quantitative studies of bird movement: a methodological review. Bird Study 46:S289–S298. doi:10.1080/00063659909477256
Pfister CA (1998) Patterns of variance in stage-structured populations: evolutionary predictions and ecological implications. Proc Natl Acad Sci USA 95:213–218
Pomeroy PP, Anderson SS, Twiss SD, McConnell BJ (1994) Dispersion and site fidelity of breeding female grey seals (Halichoerus grypus) on North Rona, Scotland. J Zool 233:429–447. doi:10.1111/j.1469-7998.1994.tb05275.x
Proffitt KM, Garrott RA, Rotella JJ (2008) Long-term evaluation of body mass at weaning and postweaning survival rates of Weddell seals in Erebus Bay, Antarctica. Mar Mammal Sci 24:677–689. doi:10.1111/j.1748-7692.2008.00207.x
R Development Core Team (2012) R: A language and environment for statistical computing. R Foundation for Statistical Computing. Vienna, Austria. ISBN 3-900051-07-0. URL http://www.R-project.org
Reid JM, Bignal EM, Bignal S, McCracken DI, Monaghan P (2003) Environmental variability, life-history covariation and cohort effects in the red-billed chough Pyrrhocorax pyrrhocorax. J Anim Ecol 72:36–46. doi:10.1046/j.1365-2656.2003.00673.x
Remy J-P, Becquevort S, Haskell TG, Tison J-L (2008) Impact of the B-15 iceberg “stranding event” on the physical and biological properties of sea ice in McMurdo Sound, Ross Sea, Antarctica. Antarct Sci 20:593–604. doi:10.1017/S0954102008001284
Rivalan P, Prévot-Julliard A-C, Choquet R, Pradel R, Jacquemin B, Girondot M (2005) Trade-off between current reproductive effort and delay to next reproduction in the leatherback sea turtle. Oecologia 145:564–574. doi:10.1007/s00442-005-0159-4
Rödel HG, von Holst D, Kraus C (2009) Family legacies: short- and long-term fitness consequences of early-life conditions in female European rabbits. J Anim Ecol 78:789–797. doi:10.1111/j.1365-2656.2009.01537.x
Rotella JJ, Link WA, Nichols JD, Hadley GL, Garrott RA, Proffitt KM (2009) An evaluation of density-dependent and density-independent influences on population growth rates in Weddell seals. Ecology 90:975–984. doi:10.1890/08-0971.1
Rotella JJ, Link WA, Chambert T, Stauffer GE, Garrott RA (2012) Evaluating the demographic buffering hypothesis with vital rates estimated for Weddell seals from 30 years of mark–recapture data. J Anim Ecol 81:162–173. doi:10.1111/j.1365-2656.2011.01902.x
Schaub M, Gimenez O, Schmidt BR, Pradel R (2004) Estimating survival and temporary emigration in the multistate capture-recapture framework. Ecology 85:2107–2113. doi:10.1890/03-3110
Schjørring S, Gregersen J, Bregnballe T (1999) Prospecting enhances breeding success of first-time breeders in the great cormorant, Phalacrocorax carbo sinensis. Anim Behav 57:647–654. doi:10.1006/anbe.1998.0993
Schwarz CJ, Stobo WT (1997) Estimating temporary migration using the robust design. Biometrics 53:178–194
Siniff DB, DeMaster DP, Hofman RJ, Eberhardt LL (1977) An analysis of the dynamics of a Weddell seal population. Ecol Monogr 47:319–335. doi:10.2307/1942520
Stirling I (1969) Ecology of the Weddell seal in McMurdo Sound, Antarctica. Ecology 50:573–586. doi:10.2307/1936247
Sydeman WJ, Huber HR, Emslie SD, Ribic CA, Nur N (1991) Age-specific weaning success of northern elephant seals in relation to previous breeding experience. Ecology 72:2204–2217. doi:10.2307/1941571
Testa JW (1994) Over-winter movements and diving behavior of female Weddell seals (Leptonychotes weddellii) in the southwestern Ross Sea, Antarctica. Can J Zool 72:1700–1710. doi:10.1139/z94-229
Testa JW, Siniff DB (1987) Population dynamics of Weddell seals (Leptonychotes weddellii) in McMurdo Sound, Antarctica. Ecol Monogr 57:149–165. doi:10.2307/1942622
Testa JW, Siniff DB, Ross MJ, Winter JD (1985) Weddell seal—Antarctic cod interactions in McMurdo Sound. In: Siegfried WR, Condy PR, Laws RM (eds) Antarctic nutrient cycles and food webs. Proceedings of the 4th SCAR Symposium on Antarctic Biology. Springer, New York, pp 561–565
White GC, Burnham KP (1999) Program MARK: survival estimation from populations of marked animals. Bird Study 46 (Suppl):120–138
White PJ, Davis TL, Barnowe-Meyer KK, Crabtree RL, Garrott RA (2007) Partial migration and philopatry of Yellowstone pronghorn. Biol Conserv 135:502–510. doi:10.1016/j.biocon.2006.10.041
Williams BK, Nichols JD, Conroy MJ (2002) Analysis and management of animal populations; modeling, estimation, and decision making. Academic, San Diego
Acknowledgments
This work was supported by the National Science Foundation, Office of Polar Programs (grant no. ANT-0635739 to R.A. Garrott, J.J. Rotella, and D.B. Siniff, and previous grants to D.B. Siniff and J.W. Testa). Animal handling protocol was approved by Montana State University’s Institutional Animal Care and Use Committee (Protocol #41-05) and complied with the Marine Mammal Permit Act of the USA and the multinational Antarctic Conservation Act. Raytheon Polar Services Corporation, Petroleum Helicopters International, and the New York National Guard provided logistical support for field work in Antarctica. We thank D.B. Siniff for numerous conversations about Weddell seal ecology and helpful advice throughout this work, W.L. Kendall for advice on analysis and critical review of previous manuscript drafts, and J.L. Laake for help with RMark coding. We appreciate the dozens of field assistants that supported this project through the years. Two anonymous reviewers provided helpful comments on an earlier manuscript draft.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Helene Marsh.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Stauffer, G.E., Rotella, J.J. & Garrott, R.A. Variability in temporary emigration rates of individually marked female Weddell seals prior to first reproduction. Oecologia 172, 129–140 (2013). https://doi.org/10.1007/s00442-012-2472-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-012-2472-z