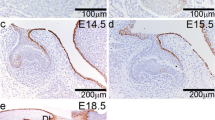
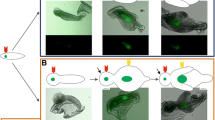
Abstract
We have previously demonstrated that tooth size is determined by dental mesenchymal factors. Exogenous bone morphogenetic protein (BMP)4, Noggin, fibroblast growth factor (FGF)3 and FGF10 have no effect on tooth size, despite the expressions of Bmp2, Bmp4, Fgf3, Fgf10 and Lef1 in the dental mesenchyme. Among the wingless (Wnt) genes that are differentially expressed during tooth development, only Wnt5a is expressed in the dental mesenchyme. The aims of the present study were to clarify the expression pattern of Wnt5a in developing tooth germs and the role of Wnt5a in the regulation of tooth size by treatment with exogenous WNT5A with/without an apoptosis inhibitor on in vitro tooth germs combined with transplantation into kidney capsules. Wnt5a was intensely expressed in both the dental epithelium and mesenchyme during embryonic days 14–17, overlapping partly with the expressions of both Shh and Bmp4. Moreover, WNT5A retarded the development of tooth germs by markedly inducing cell death in the non-dental epithelium and mesenchyme but not widely in the dental region, where the epithelial–mesenchymal gene interactions among Wnt5a, Fgf10, Bmp4 and Shh might partly rescue the cells from death in the WNT5A-treated tooth germ. Together, these results indicate that WNT5A-induced cell death inhibited the overall development of the tooth germ, resulting in smaller teeth with blunter cusps after tooth-germ transplantation. Thus, it is suggested that Wnt5a is involved in regulating cell death in non-dental regions, while in the dental region it acts as a regulator of other genes that rescue tooth germs from cell death.






Similar content being viewed by others
References
Aberg T, Wozney J, Thesleff I (1997) Expression patterns of bone morphogenetic proteins (Bmps) in the developing mouse tooth suggest roles in morphogenesis and cell differentiation. Dev Dyn 210:383–396
Cai J, Cho SW, Kim JY, Lee MJ, Cha YG, Jung HS (2007) Patterning the size and number of tooth and its cusps. Dev Biol 304:499–507
Chen Y, Bei M, Woo I, Satokata I, Maas R (1996) Msx1 controls inductive signaling in mammalian tooth morphogenesis. Development 122:3035–3044
Chen Z, Zhang L, Wang ZF, Sun ZJ, Zhang Q, Fan B (2003) Expression of Shh, Ptc1, Ptc2 mRNA in the cap stage of mouse molar. Zhonghua Kou Qiang Yi Xue Za Zhi 38:93–95
Chiang C, Swan RZ, Grachtchouk M, Bolinger M, Litingtung Y, Robertson EK, Cooper MK, Gaffield W, Westphal H, Beachy PA, Dlugosz AA (1999) Essential role for Sonic hedgehog during hair follicle morphogenesis. Dev Biol 205:1–9
Chun JS, Oh H, Yang S, Park M (2008) Wnt signaling in cartilage development and degeneration. BMB Rep 41:485–494
Dassule HR, McMahon AP (1998) Analysis of epithelial-mesenchymal interactions in the initial morphogenesis of the mammalian tooth. Dev Biol 202:215–227
Dassule HR, Lewis P, Bei M, Maas R, McMahon AP (2000) Sonic hedgehog regulates growth and morphogenesis of the tooth. Development 127:4775–4785
Eisenberg LM, Eisenberg CA (2007) Evaluating the role of Wnt signal transduction in promoting the development of the heart. ScientificWorldJournal 7:161–176
Gritli-Linde A, Bei M, Maas R, Zhang XM, Linde A, McMahon AP (2002) Shh signaling within the dental epithelium is necessary for cell proliferation, growth and polarization. Development 129:5323–5337
Hardcastle Z, Mo R, Hui CC, Sharpe PT (1998) The Shh signalling pathway in tooth development: defects in Gli2 and Gli3 mutants. Development 125:2803–2811
Jernvall J, Thesleff I (2000) Reiterative signaling and patterning during mammalian tooth morphogenesis. Mech Dev 92:19–29
Jernvall J, Aberg T, Kettunen P, Keranen S, Thesleff I (1998) The life history of an embryonic signaling center: BMP-4 induces p21 and is associated with apoptosis in the mouse tooth enamel knot. Development 125:161–169
Kettunen P, Laurikkala J, Itaranta P, Vainio S, Itoh N, Thesleff I (2000) Associations of FGF-3 and FGF-10 with signaling networks regulating tooth morphogenesis. Dev Dyn 219:322–332
Kratochwil K, Dull M, Farinas I, Galceran J, Grosschedl R (1996) Lef1 expression is activated by BMP-4 and regulates inductive tissue interactions in tooth and hair development. Genes Dev 10:1382–1394
Li C, Xiao J, Hormi K, Borok Z, Minoo P (2002) Wnt5a participates in distal lung morphogenesis. Dev Biol 248:68–81
Lin M, Li L, Liu C, Liu H, He F, Yan F, Zhang Y, Chen Y (2011) Wnt5a regulates growth, patterning, and odontoblast differentiation of developing mouse tooth. Dev Dyn 240:432–440
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods 25:402–408
Luukko K, Loes S, Furmanek T, Fjeld K, Kvinnsland IH, Kettunen P (2003) Identification of a novel putative signaling center, the tertiary enamel knot in the postnatal mouse molar tooth. Mech Dev 120:270–276
McMahon AP, Bradley A (1990) The Wnt-1 (int-1) proto-oncogene is required for development of a large region of the mouse brain. Cell 62:1073–1085
Milat F, Ng KW (2009) Is Wnt signalling the final common pathway leading to bone formation? Mol Cell Endocrinol 310:52–62
Miller JR (2002) The Wnts. Genome Biol 3:REVIEWS3001
Parr BA, McMahon AP (1995) Dorsalizing signal Wnt-7a required for normal polarity of D-V and A-P axes of mouse limb. Nature 374:350–353
Pispa J, Thesleff I (2003) Mechanisms of ectodermal organogenesis. Dev Biol 262:195–205
Reddy S, Andl T, Bagasra A, Lu MM, Epstein DJ, Morrisey EE, Millar SE (2001) Characterization of Wnt gene expression in developing and postnatal hair follicles and identification of Wnt5a as a target of Sonic hedgehog in hair follicle morphogenesis. Mech Dev 107:69–82
Sarkar L, Sharpe PT (1999) Expression of Wnt signalling pathway genes during tooth development. Mech Dev 85:197–200
Sarkar L, Sharpe PT (2000) Inhibition of Wnt signaling by exogenous Mfrzb1 protein affects molar tooth size. J Dent Res 79:920–925
Stark K, Vainio S, Vassileva G, McMahon AP (1994) Epithelial transformation of metanephric mesenchyme in the developing kidney regulated by Wnt-4. Nature 372:679–683
St-Jacques B, Dassule HR, Karavanova I, Botchkarev VA, Li J, Danielian PS, McMahon JA, Lewis PM, Paus R, McMahon AP (1998) Sonic hedgehog signaling is essential for hair development. Curr Biol 8:1058–1068
Suomalainen M, Thesleff I (2010) Patterns of Wnt pathway activity in the mouse incisor indicate absence of Wnt/beta-catenin signaling in the epithelial stem cells. Dev Dyn 239:364–372
Suzuki K, Bachiller D, Chen YP, Kamikawa M, Ogi H, Haraguchi R, Ogino Y, Minami Y, Mishina Y, Ahn K, Crenshaw EB 3rd, Yamada G (2003) Regulation of outgrowth and apoptosis for the terminal appendage: external genitalia development by concerted actions of BMP signaling [corrected]. Development 130:6209–6220
Vainio S, Karavanova I, Jowett A, Thesleff I (1993) Identification of BMP-4 as a signal mediating secondary induction between epithelial and mesenchymal tissues during early tooth development. Cell 75:45–58
Yamaguchi TP, Bradley A, McMahon AP, Jones S (1999) A Wnt5a pathway underlies outgrowth of multiple structures in the vertebrate embryo. Development 126:1211–1223
Yamamoto H, Cho SW, Kim EJ, Kim JY, Fujiwara N, Jung HS (2004) Developmental properties of the Hertwig's epithelial root sheath in mice. J Dent Res 83:688–692
Yamashiro T, Tummers M, Thesleff I (2003) Expression of bone morphogenetic proteins and Msx genes during root formation. J Dent Res 82:172–176
Zhang Y, Zhang Z, Zhao X, Yu X, Hu Y, Geronimo B, Fromm SH, Chen YP (2000) A new function of BMP4: dual role for BMP4 in regulation of Sonic hedgehog expression in the mouse tooth germ. Development 127:1431–1443
Author information
Authors and Affiliations
Corresponding author
Additional information
This research was supported by the Bio & Medical Technology Development Program of the National Research Foundation (NRF) funded by the Korean government (MEST) (No. 2011-0027790)
Jinglei Cai and Noriko Mutoh contributed equality to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplemental figure 1
Apoptosis assay of exogenous WNT5A (100 μg/ml). Hematoxylin and eosin (a, b, e, f, i, j) and TUNEL (c, d, g, h, k, l) staining in frontal sections of tooth germs cultured with PBS- (a, c, e, g, i, k) and WNT5A-soaked (b, d, f, h, j, l) beads (*) during 12 (a–d), 24 (e–h), and 48 h (i–l). c, g, k A few TUNEL-positive spots are located in the epithelium and mesenchyme of control explants. d, h, l TUNEL-labeled mesenchymal cells are observed in the area around the beads in a WNT5A-treated tooth germ. However, few TUNEL-labeled cells are detected in the dental region (arrows or arrowheads). (JPG 85 KB)
Rights and permissions
About this article
Cite this article
Cai, J., Mutoh, N., Shin, JO. et al. Wnt5a plays a crucial role in determining tooth size during murine tooth development. Cell Tissue Res 345, 367–377 (2011). https://doi.org/10.1007/s00441-011-1224-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-011-1224-4