Abstract
Less invasive surfactant therapies (LIST) use surfactant instillation through a thin tracheal catheter in spontaneously breathing infants. This review and meta-analysis investigates respiratory outcomes for preterm infants with respiratory distress syndrome treated with LIST rather than administration of surfactant through an endotracheal tube. Randomised controlled trial (RCT) full texts provided outcome data for bronchopulmonary dysplasia (BPD), death or BPD, early CPAP failure, invasive ventilation requirements and usual neonatal morbidities. Relative risks (RR) from pooled data, with subgroup analyses, were obtained from a Mantel-Haenszel analysis using a random effect model. Six RCTs evaluated LIST: 4 vs InSurE and 1 each vs delayed or immediate intubation for surfactant. LIST resulted in decreased risks of BPD (RR = 0.71 [0.52–0.99]; NNT = 21), death or BPD (RR = 0.74 [0.58–0.94]; NNT = 15) and early CPAP failure or invasive ventilation requirements (RR = 0.67 [0.53–0.84]; NNT = 8 and RR = 0.69 [0.53–0.88]; NNT = 6). Compared to InSurE, LIST decreased the risks of BPD or death (RR = 0.63 [0.44–0.92]; NNT = 11) and of early CPAP failure (RR = 0.71 [0.53–0.96]; NNT = 11). Common neonatal morbidities were not different.
Conclusions: Respiratory management with LIST decreases the risks of BPD and BPD or death, and the need for invasive ventilation. This strategy appears safe, but long-term follow-up is lacking.
What is Known: • Initial management of preterm infants with CPAP decreases the risk of death or BPD, but many still require surfactant or invasive ventilation. • Surfactant can be instilled through a tracheal thin catheter while the infant breathes on CPAP, but improvement in BPD is inconsistent between studies. |
What is New: • Less invasive surfactant therapy (LIST) strategies decrease the risks of BPD, of death or BPD, and of CPAP failure compared to strategies where surfactant is administered through an endotracheal tube. • LIST strategies decrease the risks of the composite outcome of BPD or death and of early CPAP failure when compared to “intubation-surfactant-extubation” approaches. |


Similar content being viewed by others
Abbreviations
- AMV:
-
Avoidance of Mechanical Ventilation Study
- BPD:
-
Bronchopulmonary dysplasia (here: moderate to severe)
- cPVL:
-
Cystic periventricular leucomalacia
- InSurE:
-
Intubation-surfactant-extubation
- IVH:
-
Intraventricular haemorrhage
- LISA:
-
Less invasive surfactant administration
- LIST:
-
Less invasive surfactant therapy
- MV:
-
Mechanical ventilation
- MIST:
-
Minimally invasive surfactant therapy
- nCPAP:
-
Nasal continuous positive airway pressure
- NEC:
-
Necrotising enterocolitis
- NNT/H:
-
Numbers needed to treat/to harm
- NINSAP:
-
Nonintubated Surfactant Application Study
- PDA:
-
Patent ductus arteriosus
- RCT:
-
Randomised controlled trial
- ROP:
-
Retinopathy of prematurity
- RR:
-
Relative risk
References
Bao Y, Zhang G, Wu M, Ma L, Zhu J (2015) A pilot study of less invasive surfactant administration in very preterm infants in a Chinese tertiary center. BMC Pediatr 15:–21. doi:10.1186/s12887-015-0342-7
Bohlin K, Bouhafs RK, Jarstrand C, Curstedt T, Blennow M, Robertson B (2005) Spontaneous breathing or mechanical ventilation alters lung compliance and tissue association of exogenous surfactant in preterm newborn rabbits. Pediatr Res 57(5 Pt 1):624–630. doi:10.1203/01.pdr.0000156502.84909.bc
Brix N, Sellmer A, Jensen MS, Pedersen LV, Henriksen TB (2014) Predictors for an unsuccessful INtubation-SURfactant-Extubation procedure: a cohort study. BMC Pediatr 14. doi:10.1186/1471-2431-14-155
Dargaville PA, Aiyappan A, Cornelius A, Williams C, De Paoli AG (2011) Preliminary evaluation of a new technique of minimally invasive surfactant therapy. Arch Dis Child Fetal Neonatal Ed 96(4):F243–F248. doi:10.1136/adc.2010.192518
Dargaville PA, Kamlin CO, De Paoli AG, Carlin JB, Orsini F, Soll RF, Davis PG (2014) The OPTIMIST-A trial: evaluation of minimally-invasive surfactant therapy in preterm infants 25-28 weeks gestation. BMC Pediatr 14:213. doi:10.1186/1471-2431-14-213
Dekker J, Lopriore E, Rijken M, Rijntjes-Jacobs E, Smits-Wintjens V, Te Pas A (2016) Sedation during minimal invasive surfactant therapy in preterm infants. Neonatology 109(4):308–313. doi:10.1159/000443823
Dunn MS, Kaempf J, de Klerk A, de Klerk R, Reilly M, Howard D, Ferrelli K, O’Conor J, Soll RF (2011) Randomized trial comparing 3 approaches to the initial respiratory management of preterm neonates. Pediatrics 128(5):e1069–e1076. doi:10.1542/peds.2010-3848
Fischer HS, Buhrer C (2013) Avoiding endotracheal ventilation to prevent bronchopulmonary dysplasia: a meta-analysis. Pediatrics 132(5):e1351–e1360. doi:10.1542/peds.2013-1880
Göpel W, Kribs A, Ziegler A, Laux R, Hoehn T, Wieg C, Siegel J, Avenarius S, von der Wense A, Vochem M, Groneck P, Weller U, Moller J, Hartel C, Haller S, Roth B, Herting E (2011) Avoidance of mechanical ventilation by surfactant treatment of spontaneously breathing preterm infants (AMV): an open-label, randomised, controlled trial. Lancet 378(9803):1627–1634. doi:10.1016/s0140-6736(11)60986-0
Göpel W, Kribs A, Hartel C, Avenarius S, Teig N, Groneck P, Olbertz D, Roll C, Vochem M, Weller U, von der Wense A, Wieg C, Wintgens J, Preuss M, Ziegler A, Roth B, Herting E (2015) Less invasive surfactant administration is associated with improved pulmonary outcomes in spontaneously breathing preterm infants. Acta Paediatr 104(3):241–246. doi:10.1111/apa.12883
Heidarzadeh M, Mirnia K, Hoseini MB, Sadeghnia A, Akrami F, Balila M, Ghojazadeh M, Shafai F (2013) Surfactant administration via thin catheter during spontaneous breathing: randomized controlled trial in Alzahra hospital. Iranian Journal of Neonataology 4(2):5–9
Hillman NH, Moss TJ, Nitsos I, Jobe AH (2012) Moderate tidal volumes and oxygen exposure during initiation of ventilation in preterm fetal sheep. Pediatr Res 72(6):593–599. doi:10.1038/pr.2012.135
Ioannidis JP, Horbar JD, Ovelman CM, Brosseau Y, Thorlund K, Buus-Frank ME, Mills EJ, Soll RF (2015) Completeness of main outcomes across randomized trials in entire discipline: survey of chronic lung disease outcomes in preterm infants. BMJ (Clinical research ed) 350:h72. doi:10.1136/bmj.h72
Isayama T, Chai-Adisaksopha C, McDonald SD (2015) Noninvasive ventilation with vs without early surfactant to prevent chronic lung disease in preterm infants: a systematic review and meta-analysis. JAMA Pediatr 169(8):731–739. doi:10.1001/jamapediatrics.2015.0510
Isayama T, Iwami H, McDonald S, Beyene J (2016) Association of noninvasive ventilation strategies with mortality and bronchopulmonary dysplasia among preterm infants: a systematic review and meta-analysis. JAMA : the journal of the American Medical Association 316(6):611–624. doi:10.1001/jama.2016.10708
Kanmaz HG, Erdeve O, Canpolat FE, Mutlu B, Dilmen U (2013) Surfactant administration via thin catheter during spontaneous breathing: randomized controlled trial. Pediatrics 131(2):e502–e509. doi:10.1542/peds.2012-0603
Kribs A, Pillekamp F, Hunseler C, Vierzig A, Roth B (2007) Early administration of surfactant in spontaneous breathing with nCPAP: feasibility and outcome in extremely premature infants (postmenstrual age </=27 weeks). Paediatr Anaesth 17(4):364–369. doi:10.1111/j.1460-9592.2006.02126.x
Kribs A, Roll C, Gopel W, Wieg C, Groneck P, Laux R, Teig N, Hoehn T, Bohm W, Welzing L, Vochem M, Hoppenz M, Buhrer C, Mehler K, Stutzer H, Franklin J, Stohr A, Herting E, Roth B (2015) Nonintubated surfactant application vs conventional therapy in extremely preterm infants: a randomized clinical trial. JAMA Pediatr 169(8):723–730. doi:10.1001/jamapediatrics.2015.0504
Mirnia K, Heidarzadeh M, Hoseini MB, Sadeghnia AR, Balila M, Ghojazadeh M (2013) Comparison outcome of surfactant administration via tracheal catheterization during spontaneous breathing with INSURE. Med J Islam World Acad Sci 21(4):143–148
Mohammadizadeh M, Ardestani AG, Sadeghnia AR (2015) Early administration of surfactant via a thin intratracheal catheter in preterm infants with respiratory distress syndrome: feasibility and outcome. Journal of research in pharmacy practice 4(1):31–36. doi:10.4103/2279-042x.150053
Niemarkt HJ, Kuypers E, Jellema R, Ophelders D, Hutten M, Nikiforou M, Kribs A, Kramer BW (2014) Effects of less-invasive surfactant administration on oxygenation, pulmonary surfactant distribution, and lung compliance in spontaneously breathing preterm lambs. Pediatr Res 76(2):166–170. doi:10.1038/pr.2014.66
Oncel MY, Arayici S, Uras N, Alyamac-Dizdar E, Sari FN, Karahan S, Canpolat FE, Oguz SS, Dilmen U (2016) Nasal continuous positive airway pressure versus nasal intermittent positive-pressure ventilation within the minimally invasive surfactant therapy approach in preterm infants: a randomised controlled trial. Arch Dis Child Fetal Neonatal Ed 101(4):F323–F328. doi:10.1136/archdischild-2015-308204
Porath M, Korp L, Wendrich D, Dlugay V, Roth B, Kribs A (2011) Surfactant in spontaneous breathing with nCPAP: neurodevelopmental outcome at early school age of infants <= 27 weeks. Acta Paediatr 100(3):352–359. doi:10.1111/j.1651-2227.2010.02068.x
Ramanathan R, Rasmussen MR, Gerstmann DR, Finer N, Sekar K (2004) A randomized, multicenter masked comparison trial of poractant alfa (Curosurf) versus beractant (Survanta) in the treatment of respiratory distress syndrome in preterm infants. Am J Perinatol 21(3):109–119. doi:10.1055/s-2004-823779
Rigo, V, Debauche, C, Maton, P, Broux, I, Van Laere, D (2016) Devices for less invasive surfactant therapy: a manikin study [Abstract, poster]. For presentation at the 6th Congress of the European Academy of Paediatric Societies, Geneva, Switzerland, October 21–25
Sandri F, Plavka R, Simeoni U (2008) The CURPAP study: an international randomized controlled trial to evaluate the efficacy of combining prophylactic surfactant and early nasal continuous positive airway pressure in very preterm infants. Neonatology 94(1):60–62. doi:10.1159/000113060
Schmolzer GM, Kumar M, Pichler G, Aziz K, O’Reilly M, Cheung PY (2013) Non-invasive versus invasive respiratory support in preterm infants at birth: systematic review and meta-analysis. BMJ (Clinical research ed) 347:f5980. doi:10.1136/bmj.f5980
Stevens, TP, Harrington, EW, Blennow, M, Soll, RF (2007) Early surfactant administration with brief ventilation vs. selective surfactant and continued mechanical ventilation for preterm infants with or at risk for respiratory distress syndrome. Cochrane Database Syst Rev (4):CD003063. doi:10.1002/14651858.CD003063.pub3
Subramaniam, P, Ho, JJ, Davis, PG (2016) Prophylactic nasal continuous positive airway pressure for preventing morbidity and mortality in very preterm infants. Cochrane Database Syst Rev (6):CD001243. doi:10.1002/14651858.CD001243.pub3
Teig N, Weitkamper A, Rothermel J, Bigge N, Lilienthal E, Rossler L, Hamelmann E (2015) Observational study on less invasive surfactant administration (LISA) in preterm infants <29 weeks—short and long-term outcomes. Zeitschrift fur Geburtshilfe und Neonatologie 219(6):266–273. doi:10.1055/s-0035-1547295
Verder H, Agertoft L, Albertsen P, Christensen NC, Curstedt T, Ebbesen F, Greisen G, Hobolth N, Holm V, Jacobsen T et al (1992) Surfactant treatment of newborn infants with respiratory distress syndrome primarily treated with nasal continuous positive air pressure. A pilot study. Ugeskr Laeger 154(31):2136–2139
Wallace MJ, Probyn ME, Zahra VA, Crossley K, Cole TJ, Davis PG, Morley CJ, Hooper SB (2009) Early biomarkers and potential mediators of ventilation-induced lung injury in very preterm lambs. Respir Res 10:19. doi:10.1186/1465-9921-10-19
Acknowledgments
We thank Professor Filip Cools (AZ-VUB, Brussels) for his helpful comments on a previous version of this manuscript. We thank Mr. Luc Hourlay (KCE, Centre fédéral d’expertise des soins de santé, Brussels) for his assistance in the EMBASE search.
Authors’ Contributions
VR designed the study, analysed the data and wrote the initial draft of the manuscript. VR and CL performed the literature search. VR and IB evaluated the RCTs and extracted the data. All the authors interpreted the results and revised and approved the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
VR has received speaker honoraria and sponsoring to attend a scientific meeting from Chiesi Belgium, a surfactant-producing company. The company was not involved in this study. CL and IB declare having no conflict of interest.
Financial support
None.
Ethical approval
This article does not contain any study with human participants performed by any of the authors. All studies included in the meta-analysis were approved by ethical review boards and requested parental consent.
Additional information
Communicated by Patrick Van Reempts
Electronic supplementary material
Online Resource 1
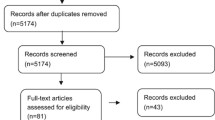
Figure: Flow diagram for selection of eligible studies. (PDF 224 kb)
Online Resource 2
Table: Risk of bias assessment. (PDF 41 kb)
Online Resource 3
Figure: Forest plots for each dichotomous outcomes. (PDF 318 kb)
Online Resource 4
Figure: Forest plots for infants born below 29 weeks. (PDF 124 kb)
Online Resource 5
Table: Data for continuous outcomes. (PDF 55 kb)
Rights and permissions
About this article
Cite this article
Rigo, V., Lefebvre, C. & Broux, I. Surfactant instillation in spontaneously breathing preterm infants: a systematic review and meta-analysis. Eur J Pediatr 175, 1933–1942 (2016). https://doi.org/10.1007/s00431-016-2789-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00431-016-2789-4