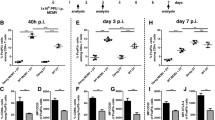
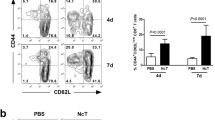
Abstract
Natural killer (NK) and CD8+ T cells play a crucial role in the control of mouse cytomegalovirus (MCMV) infection. These effector cells exert their functions by releasing antiviral cytokines and by cytolytic mechanisms including perforin activation. In addition to their role in virus control, NK cells play an immunoregulatory role since they shape the CD8+ T cell response to MCMV. To investigate the role of perforin-dependent cytolytic mechanism in NK cell modulation of CD8+ T cell response during acute MCMV infection, we have used perforin-deficient C57BL/6 mice (Prf1−/−) and have shown that virus control by CD8+ T cells in Prf1−/− mice is more efficient if NK cells are activated by the engagement of the Ly49H receptor with the m157 MCMV protein. A lack of perforin results in severe liver inflammation after MCMV infection, which is characterized by immunopathological lesions that are more pronounced in Prf1−/− mice infected with virus unable to activate NK cells. This immunopathology is caused by an abundant infiltration of activated CD8+ T cells. The depletion of CD8+ T cells has markedly reduced pathohistological lesions in the liver and improved the survival of Prf1−/− mice in spite of an increased viral load. Altogether, the results of our study suggest that a lack of perforin and absence of the specific activation of NK cells during acute MCMV infection lead to an unleashed CD8+ T cell response that is detrimental for the host.





Similar content being viewed by others
References
Pass RF (2001) Cytomegalovirus. In: Knipe DM, Howley PM (eds) Fields virology, vol 2, 4th edn. Lippincott Williams and Wilkins, Philadelphia, pp 2675–2706
Krmpotic A, Bubic I, Polic B, Lucin P, Jonjic S (2003) Pathogenesis of murine cytomegalovirus infection. Microbes Infect 5(13):1263–1277. doi:10.1016/j.micinf.2003.09.007
Reddehase MJ, Podlech J, Grzimek NK (2002) Mouse models of cytomegalovirus latency: overview. J Clin Virol 25(Suppl 2):S23–S36. doi:10.1016/S1386-6532(02)00087-2
Jonjic S, Bubic I, Krmpotic A (2006) Innate immunity to cytomegaloviruses. In: Reddehase MJ (ed) Cytomegaloviruses: molecular biology and immunology. Caister Academic Press, Wymondham, pp 285–319
Curtsinger JM, Johnson CM, Mescher MF (2003) CD8 T cell clonal expansion and development of effector function require prolonged exposure to antigen, costimulation, and signal 3 cytokine. J Immunol 171(10):5165–5171. doi:10.4049/jimmunol.171.10.5165
Curtsinger JM, Valenzuela JO, Agarwal P, Lins D, Mescher MF (2005) Type I IFNs provide a third signal to CD8 T cells to stimulate clonal expansion and differentiation. J Immunol 174(8):4465–4469. doi:10.4049/jimmunol.174.8.4465
French AR, Yokoyama WM (2003) Natural killer cells and viral infections. Curr Opin Immunol 15(1):45–51. doi:10.1016/S095279150200002X
Trinchieri G (2003) Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat Rev Immunol 3(2):133–146. doi:10.1038/nri1001
Lodoen MB, Lanier LL (2006) Natural killer cells as an initial defense against pathogens. Curr Opin Immunol 18(4):391–398. doi:10.1016/j.coi.2006.05.002
Arase H, Mocarski ES, Campbell AE, Hill AB, Lanier LL (2002) Direct recognition of cytomegalovirus by activating and inhibitory NK cell receptors. Science 296(5571):1323–1326. doi:10.1126/science.1070884
Bubic I, Wagner M, Krmpotic A, Saulig T, Kim S, Yokoyama WM, Jonjic S, Koszinowski UH (2004) Gain of virulence caused by loss of a gene in murine cytomegalovirus. J Virol 78(14):7536–7544. doi:10.1128/JVI.78.14.7536-7544.2004
Smith HR, Heusel JW, Mehta IK, Kim S, Dorner BG, Naidenko OV, Iizuka K, Furukawa H, Beckman DL, Pingel JT, Scalzo AA, Fremont DH, Yokoyama WM (2002) Recognition of a virus-encoded ligand by a natural killer cell activation receptor. Proc Natl Acad Sci USA 99(13):8826–8831. doi:10.1073/pnas.092258599
Biron CA (2012) Yet another role for natural killer cells: cytotoxicity in immune regulation and viral persistence. Proc Natl Acad Sci USA 109(6):1814–1815. doi:10.1073/pnas.1120528109
Zingoni A, Cerboni C, Ardolino M, Santoni A (2009) Modulation of T cell-mediated immune responses by natural killer cells. In: Zimmer J (ed) Natural killer cells at the forefront of modern immunology. Springer, New York, pp 315–327. doi:10.1007/978-3-642-02309-5_17
Andrews DM, Estcourt MJ, Andoniou CE, Wikstrom ME, Khong A, Voigt V, Fleming P, Tabarias H, Hill GR, van der Most RG, Scalzo AA, Smyth MJ, Degli-Esposti MA (2010) Innate immunity defines the capacity of antiviral T cells to limit persistent infection. J Exp Med 207(6):1333–1343. doi:10.1084/jem.20091193
Lang PA, Lang KS, Xu HC, Grusdat M, Parish IA, Recher M, Elford AR, Dhanji S, Shaabani N, Tran CW, Dissanayake D, Rahbar R, Ghazarian M, Brustle A, Fine J, Chen P, Weaver CT, Klose C, Diefenbach A, Haussinger D, Carlyle JR, Kaech SM, Mak TW, Ohashi PS (2012) Natural killer cell activation enhances immune pathology and promotes chronic infection by limiting CD8+ T-cell immunity. Proc Natl Acad Sci USA 109(4):1210–1215. doi:10.1073/pnas.1118834109
Lee SH, Kim KS, Fodil-Cornu N, Vidal SM, Biron CA (2009) Activating receptors promote NK cell expansion for maintenance, IL-10 production, and CD8 T cell regulation during viral infection. J Exp Med 206(10):2235–2251. doi:10.1084/jem.20082387
Mitrovic M, Arapovic J, Jordan S, Fodil-Cornu N, Ebert S, Vidal SM, Krmpotic A, Reddehase MJ, Jonjic S (2012) The NK cell response to mouse cytomegalovirus infection affects the level and kinetics of the early CD8(+) T-cell response. J Virol 86(4):2165–2175. doi:10.1128/JVI.06042-11
Mitrovic M, Arapovic J, Traven L, Krmpotic A, Jonjic S (2012) Innate immunity regulates adaptive immune response: lessons learned from studying the interplay between NK and CD8+ T cells during MCMV infection. Med Microbiol Immunol 201(4):487–495. doi:10.1007/s00430-012-0263-0
Su HC, Nguyen KB, Salazar-Mather TP, Ruzek MC, Dalod MY, Biron CA (2001) NK cell functions restrain T cell responses during viral infections. Eur J Immunol 31(10):3048–3055. doi:10.1002/1521-4141(2001010)31:10<3048:AID-IMMU3048>3.0.CO;2-1
Soderquest K, Walzer T, Zafirova B, Klavinskis LS, Polic B, Vivier E, Lord GM, Martin-Fontecha A (2011) Cutting edge: CD8+ T cell priming in the absence of NK cells leads to enhanced memory responses. J Immunol 186(6):3304–3308. doi:10.4049/jimmunol.1004122
Robbins SH, Bessou G, Cornillon A, Zucchini N, Rupp B, Ruzsics Z, Sacher T, Tomasello E, Vivier E, Koszinowski UH, Dalod M (2007) Natural killer cells promote early CD8 T cell responses against cytomegalovirus. PLoS Pathog 3(8):e123. doi:10.1371/journal.ppat.0030123
Slavuljica I, Busche A, Babic M, Mitrovic M, Gasparovic I, Cekinovic D, Markova Car E, Pernjak Pugel E, Cikovic A, Lisnic VJ, Britt WJ, Koszinowski U, Messerle M, Krmpotic A, Jonjic S (2010) Recombinant mouse cytomegalovirus expressing a ligand for the NKG2D receptor is attenuated and has improved vaccine properties. J Clin Invest 120(12):4532–4545. doi:10.1172/JCI43961
Stadnisky MD, Xie X, Coats ER, Bullock TN, Brown MG (2011) Self MHC class I-licensed NK cells enhance adaptive CD8 T-cell viral immunity. Blood 117(19):5133–5141. doi:10.1182/blood-2010-12-324632
Ferlazzo G, Tsang ML, Moretta L, Melioli G, Steinman RM, Munz C (2002) Human dendritic cells activate resting natural killer (NK) cells and are recognized via the NKp30 receptor by activated NK cells. J Exp Med 195(3):343–351. doi:10.1084/jem.20011149
Gerosa F, Baldani-Guerra B, Nisii C, Marchesini V, Carra G, Trinchieri G (2002) Reciprocal activating interaction between natural killer cells and dendritic cells. J Exp Med 195(3):327–333. doi:10.1084/jem.20010938
Laffont S, Seillet C, Ortaldo J, Coudert JD, Guery JC (2008) Natural killer cells recruited into lymph nodes inhibit alloreactive T-cell activation through perforin-mediated killing of donor allogeneic dendritic cells. Blood 112(3):661–671. doi:10.1182/blood-2007-10-120089
Piccioli D, Sbrana S, Melandri E, Valiante NM (2002) Contact-dependent stimulation and inhibition of dendritic cells by natural killer cells. J Exp Med 195(3):335–341. doi:10.1084/jem.20010934
Li MO, Wan YY, Sanjabi S, Robertson AK, Flavell RA (2006) Transforming growth factor-beta regulation of immune responses. Annu Rev Immunol 24:99–146. doi:10.1146/annurev.immunol.24.021605.090737
Kagi D, Ledermann B, Burki K, Seiler P, Odermatt B, Olsen KJ, Podack ER, Zinkernagel RM, Hengartner H (1994) Cytotoxicity mediated by T cells and natural killer cells is greatly impaired in perforin-deficient mice. Nature 369(6475):31–37. doi:10.1038/369031a0
Wagner M, Jonjic S, Koszinowski UH, Messerle M (1999) Systematic excision of vector sequences from the BAC-cloned herpesvirus genome during virus reconstitution. J Virol 73(8):7056–7060
Jonjic S, Krmpotic A, Arapovic J, Koszinowski UH (2008) Dissection of the antiviral NK cell response by MCMV mutants. Methods Mol Biol 415:127–149. doi:10.1007/978-1-59745-570-1_8
Yokoyama WM, Kim S (2008) Analysis of individual natural killer cell responses. Methods Mol Biol 415:179–196. doi:10.1007/978-1-59745-570-1_11
Munks MW, Gold MC, Zajac AL, Doom CM, Morello CS, Spector DH, Hill AB (2006) Genome-wide analysis reveals a highly diverse CD8 T cell response to murine cytomegalovirus. J Immunol 176(6):3760–3766. doi:10.4049/jimmunol.176.6.3760
Trgovcich J, Stimac D, Polic B, Krmpotic A, Pernjak-Pugel E, Tomac J, Hasan M, Wraber B, Jonjic S (2000) Immune responses and cytokine induction in the development of severe hepatitis during acute infections with murine cytomegalovirus. Arch Virol 145(12):2601–2618
Holtappels R, Podlech J, Pahl-Seibert MF, Julch M, Thomas D, Simon CO, Wagner M, Reddehase MJ (2004) Cytomegalovirus misleads its host by priming of CD8 T cells specific for an epitope not presented in infected tissues. J Exp Med 199(1):131–136. doi:10.1084/jem.20031582
Holtappels R, Thomas D, Reddehase MJ (2009) The efficacy of antigen processing is critical for protection against cytomegalovirus disease in the presence of viral immune evasion proteins. J Virol 83(18):9611–9615. doi:10.1128/JVI.00936-09
Bannard O, Kraman M, Fearon DT (2009) Secondary replicative function of CD8+ T cells that had developed an effector phenotype. Science 323(5913):505–509. doi:10.1126/science.1166831
Boyman O, Sprent J (2012) The role of interleukin-2 during homeostasis and activation of the immune system. Nat Rev Immunol 12(3):180–190. doi:10.1038/nri3156
van Dommelen SL, Sumaria N, Schreiber RD, Scalzo AA, Smyth MJ, Degli-Esposti MA (2006) Perforin and granzymes have distinct roles in defensive immunity and immunopathology. Immunity 25(5):835–848. doi:10.1016/j.immuni.2006.09.010
Andrews DM, Andoniou CE, Fleming P, Smyth MJ, Degli-Esposti MA (2008) The early kinetics of cytomegalovirus-specific CD8+ T-cell responses are not affected by antigen load or the absence of perforin or gamma interferon. J Virol 82(10):4931–4937. doi:10.1128/JVI.02127-07
Tay CH, Welsh RM (1997) Distinct organ-dependent mechanisms for the control of murine cytomegalovirus infection by natural killer cells. J Virol 71(1):267–275
Loh J, Chu DT, O’Guin AK, Yokoyama WM, Virgin HW (2005) Natural killer cells utilize both perforin and gamma interferon to regulate murine cytomegalovirus infection in the spleen and liver. J Virol 79(1):661–667. doi:10.1128/JVI.79.1.661-667.2005
Sumaria N, van Dommelen SL, Andoniou CE, Smyth MJ, Scalzo AA, Degli-Esposti MA (2009) The roles of interferon-gamma and perforin in antiviral immunity in mice that differ in genetically determined NK-cell-mediated antiviral activity. Immunol Cell Biol 87(7):559–566. doi:10.1038/icb.2009.41
Podlech J, Holtappels R, Pahl-Seibert MF, Steffens HP, Reddehase MJ (2000) Murine model of interstitial cytomegalovirus pneumonia in syngeneic bone marrow transplantation: persistence of protective pulmonary CD8-T-cell infiltrates after clearance of acute infection. J Virol 74(16):7496–7507
Podlech J, Holtappels R, Wirtz N, Steffens HP, Reddehase MJ (1998) Reconstitution of CD8 T cells is essential for the prevention of multiple-organ cytomegalovirus histopathology after bone marrow transplantation. J Gen Virol 79(Pt 9):2099–2104
Badovinac VP, Hamilton SE, Harty JT (2003) Viral infection results in massive CD8+ T cell expansion and mortality in vaccinated perforin-deficient mice. Immunity 18(4):463–474. doi:10.1016/S1074-7613(03)00079-7
Sad S, Kagi D, Mosmann TR (1996) Perforin and Fas killing by CD8+ T cells limits their cytokine synthesis and proliferation. J Exp Med 184(4):1543–1547. doi:10.1084/jem.184.4.1543
Acknowledgments
We thank Jelena Boneta for performing immunofluorescent CD8+ T cell staining, Prof. Nives Jonjic for help with pathohistological analysis, and Dr. Felix M. Wensveen for discussion. J. A. is supported by the Federal Ministry of Education and Science, Bosnia and Herzegovina. This work has been supported in part by Croatian Science Foundation under the project 7132 (to AK) and by the University of Rijeka under the projects 13.06.1.1.01 (to SJ) and 13.06.1.1.02 (to AK).
Conflict of interest
The authors declare no financial or commercial conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Jurica Arapović and Maja Arapović have contributed equally to this work.
This article is part of the Special Issue on Cytomegalovirus.
Rights and permissions
About this article
Cite this article
Arapović, J., Arapović, M., Golemac, M. et al. The specific NK cell response in concert with perforin prevents CD8+ T cell-mediated immunopathology after mouse cytomegalovirus infection. Med Microbiol Immunol 204, 335–344 (2015). https://doi.org/10.1007/s00430-015-0409-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00430-015-0409-y