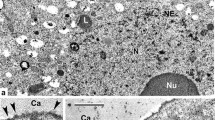
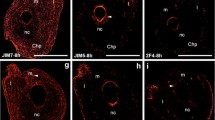
Abstract
Main conclusion
Both male and female gametes of archegoniates are highly specialized cells surrounded by an extraprotoplasmic matrix rich in AGPs, which are speculated to facilitate development and gamete fusion through Ca 2+ oscillations. An additional layer, the egg envelope, forms around the egg periphery, except at the fertilization pore, and contains arabinose-rich polymers that presumably impart flexibility for the rapidly growing zygote and embryo. The abundant AGPs and arabinan pectins associated with the eggs of C. richardii not only are integral to development, fertilization, and early embryogenesis, but also may be involved in desiccation tolerance important to the survival of the reproductive gametophyte.
A defining feature of gametogenesis in archegoniates is the deposition of a special matrix outside of the plasmalemma of both egg and sperm cells that displaces the primary cell wall away from the protoplasm. It is within this matrix that gamete differentiation occurs. In leptosporangiate ferns, maturation of the egg cell involves the deposition of a second specialized wall, the so-called egg envelope that surrounds the cell except at the fertilization pore, a narrow site where gamete fusion takes place. We provide the first conclusive evidence of the macromolecular constituents in the unique structures surrounding fern egg cells before and after fertilization. To test the hypotheses that the egg extracellular matrix contains arabinogalactan proteins (AGPs) as does the sperm cell matrix, and that cell wall polysaccharides, especially pectins, are components of the egg envelope, we examined the expression patterns of AGPs and cell wall constituents during oogenesis in Ceratopteris richardii. Utilizing histochemical stains for callose, cellulose and AGPs coupled with immunogold localizations employing a suite of monoclonal antibodies to cell wall components (JIM13, JIM8, LM2, LM5, LM6, LM19, LM20 and anticallose), we demonstrate that AGPs, but not pectins, are abundant in the matrix around egg cells and degrading neck canal and ventral canal cells during archegonial development. A striking finding is that both AGPs and (1,5)-α-l-arabinan pectin epitopes are principle components of the egg envelope before and after fertilization, suggesting that they are important in both egg maturation and gamete fusion.






Similar content being viewed by others
Abbreviations
- AGP:
-
Arabinogalactan protein
- MAb:
-
Monoclonal antibody
References
Acosta-García G, Vielle-Calzada JP (2004) A classical arabinogalactan protein is essential for the initiation of female gametogenesis in Arabidopsis. Plant Cell 16:2614–2628
Antoine AF, Faure JE, Cordeiro S, Dumas C, Rougier M, Feijo JA (2000) A calcium influx is triggered and propagates in the zygote as a wave front during in vitro fertilization of flowering plants. Proc Natl Acad Sci 97:10643–10648
Bao WM, Qun HE, Wang QX, Tian GW, Cao JG (2005) Ultrastructure of oogenesis in Dryopteris crassirhizoma Nakai. J Integr Plant Biol 47:201–213
Bell PR, Duckett JG (1976) Gametogenesis and fertilization in Pteridium. Bot J Linn Soc 73:47–78
Bush MS, Marry M, Huxham MI, Jarvis MC, McCann MC (2001) Developmental regulation of pectic epitopes during potato tuberisation. Planta 213:869–880
Cao JG, Wang QX, Bao WM (2010a) Formation of the fertilization pore during oogenesis of the fern Ceratopteris thalictroides. J Integr Plant Biol 52:518–527
Cao JG, Wang QX, Dai XL (2010b) Ultrastructural observations of oogenesis in the fern Adiantum flabellulatum L. (Adiantaceae). Am Fern J 100:93–102
Cao JG, Dai XL, Wang QX (2012a) Cytological features of oogenesis and their evolutionary significance in the fern Osmunda japonica. Sex Plant Reprod 25:147–156
Cao JG, Dai XL, Wang QX (2012b) Ultrastructural and chemical studies on oogenesis of the fern Pteridium aquilinum. Sex Plant Reprod 25:61–69
Cave CF, Bell PR (1974) The nature of the membrane around the egg of Pteridium aquilinum (L.) Kuhn. Ann Bot 38:17–21
Chudzik B, Zarzyka B, Śniezko R (2005) Immunodetection of arabinogalactan proteins in different types of plant ovules. Acta Biol Cracov Ser Bot 47:139–146
Coimbra S, Duarte C (2003) Arabinogalactan proteins may facilitate the movement of pollen tubes from the stigma to the ovules in Actinidia deliciosa and Amaranthus hypochondriacus. Euphytica 133:171–178
Coimbra S, Pereira G (2012) Arabinogalactan proteins in Arabidopsis thaliana pollen development. In: Ciftci YO (ed) Transgenic plants—advances and limitations, pp 329–352, InTech. http://www.intechopen.com/books/transgenic-plants-advances-and-limitations/arabinogalactan-proteins-in-arabidopsis-thaliana-pollen-development
Coimbra S, Almeida J, Junqueira V, Costa ML, Pereira LG (2007) Arabinogalactin proteins as a molecular markers in Arabidopsis thaliana sexual reproduction. J Exp Bot 58:4027–4035
Coimbra S, Jones B, Pereira LG (2008) Arabinogalactan proteins (AGPs) related to pollen tube guidance into the embryo sac in Arabidopsis. Plant Signal Behav 3:455–456
Costa M, Pereira AM, Rudall PJ, Coimbra S (2013) Immunolocalization of arabinogalactan proteins (AGPs) in reproductive structures of an early-divergent angiosperm, Trithuria (Hydatellaceae). Ann Bot Lond 111:183–190
Denninger P, Bleckmann A, Lausser A, Vogler F, Ott T, Ehrhardt DW, Frommer WB, Sprunck S, Dresselhaus T, Grossman G (2014) Male–female communication triggers calcium signatures during fertilization in Arabidopsis. Nat Commun 5:1–12
Ducibella T, Huneau D, Angelichio E, Xu Z, Schultz RM, Kopf GS, Fissore R, Madoux S, Ozil J-P (2002) Egg-to-embryo transition is driven by differential responses to Ca2+ oscillation number. Dev Biol 250:280–291
Eeckhout S, Leroux O, Willats WG, Popper ZA, Viane RL (2014) Comparative glycan profiling of Ceratopteris richardii ‘C-Fern’gametophytes and sporophytes links cell-wall composition to functional specialization. Ann Bot Lond 114:1295–1307
Fangel JU, Ulvskov P, Knox JP, Mikkelsen MD, Harholt J, Popper ZA, Willats WGT (2012) Cell wall evolution and diversity. Front Plant Sci 3:1–8
Fasciati R, Schneller J, Jenni V, Roos UP (1994) Fertilization in the fern Athyrium felix-femina (Pterophyta) II. Ultrastructure. Crypt Bot 4:356–367
Faure JE, Digonnet C, Dumas C (1994) An in vitro system for adhesion and fusion of maize gametes. Science 263:1598–1600
Ge LL, Tian HQ, Russell SD (2007) Calcium function and distribution during fertilization in angiosperms. Am J Bot 94:1046–1060
Gomez LD, Steele-King CG, Jones L, Foster JM, Vuttipongchaikij S, McQueen-Mason SJ (2009) Arabinan metabolism during seed development and germination in Arabidopsis. Mol Plant 2:966–976
Ha MA, Viëtor RJ, Jardine GD, Apperley DC, Jarvis MC (2005) Conformation and mobility of the arabinan and galactan side chains of pectin. Phytochemistry 66:1817–1824
Jaffe LF (1990) The roles of intramembrane calcium in polarizing and activating eggs. In: Dale B (ed) Mechanism of fertilization: plants to humans. Springer, Berlin, pp 389–417
Jauh GY, Lord FM (1996) Localization of pectins and arabinogalactan-proteins in lily (Lilium longiflorum L.) pollen tube and style and their possible roles in pollination. Planta 199:251–261
Johnson GP, Renzaglia KS (2008) Embryology of Ceratopteris richardii (Pteridaceae, tribe Ceratopterideae), with emphasis on placental development. J Plant Res 12:581–592
Johnson GP, Renzaglia KS (2009) Evaluating the diversity of pteridophyte embryology in the light of recent phylogenetic analyses leads to new inferences on character evolution. Plant Syst Evol 283:149–164
Jones L, Seymour GB, Knox JP (1997) Localization of pectic galactan in tomato cell walls using a monoclonal antibody specific to (1–4)-β-d-galactan. Plant Physiol 113:1405–1412
Jones L, Milne JL, Ashford D, McQueen-Mason SJ (2003) Cell wall arabinan is essential for guard cell function. Proc Natl Acad Sci 100:11783–11788
Lamport DT, Várnai P (2013) Periplasmic arabinogalactan glycoproteins act as a calcium capacitor that regulates plant growth and development. New Phytol 197:58–64
Lloyd RM (1974) Systematics of the genus Ceratopteris Brongn.(Parkeriaceae) II. Taxonomy. Brittonia 26:139–160
Lopez RA, Renzaglia KS (2014) Multiflagellated sperm cells of Ceratopteris richardii are bathed in arabinogalactan proteins throughout development. Am J Bot 101:2052–2061
Lopez-Smith RA, Renzaglia KS (2008) Sperm cell architecture, insemination, and fertilization in the model fern, Ceratopteris richardii. Sex Plant Reprod 21:153–167
Majewska-Sawka A, Nothnagel EA (2000) The multiple roles of arabinogalactan proteins in plant development. Plant Physiol 122:3–10
Meikle PJ, Bonig I, Hoogenraad NJ, Clarke AE, Stone BA (1991) The location of (1–3)-β-glucans in the walls of pollen tubes of Nicotiana alata using a (1–3)-β-glucan-specific monoclonal antibody. Planta 185:1–8
Mogami N, Nakamura S, Nakamura N (1999) Immunolocalization of the cell wall components in Pinus densiflora pollen. Protoplasma 206:1–10
Moore JP, Vicré-Gibouin M, Farrant JM, Driouich A (2008) Adaptations of higher plant cell walls to water loss: drought vs desiccation. Physiol Plantarum 134:237–245
Moore JP, Nguema-Ona EE, Vicré-Gibouin M, Sørensen I, Willats WG, Driouich A, Farrant JM (2013) Arabinose-rich polymers as an evolutionary strategy to plasticize resurrection plant cell walls against desiccation. Planta 237:739–754
Myles DG (1978) The fine structure of fertilization in the fern Marsilea vestita. J Cell Sci 30:265–281
Pennell RI, Janniche L, Kjellbom P, Scofield GN, Peart JM, Roberts K (1991) Developmental regulation of a plasma membrane arabinogalactan protein epitope in oilseed rape flowers. Plant Cell 3:1317–1326
Popper ZA, Michel G, Hervé C, Domozych DS, Willats WGT, Tuohy MG, Kloareg B, Stengel DB (2011) Evolution and diversity of plant cell walls: from algae to flowering plants. Ann Rev Plant Biol 62:567–590
Qin Y, Zhao J (2006) Localization of arabinogalactan proteins in egg cells, zygotes, and two-celled proembryos and effects of β-d-glucosyl Yariv reagent on egg cell fertilization and zygote division in Nicotiana tabacum L. J Exp Bot 57:2061–2074
Rafińska K, Bednarska E (2011) Localisation pattern of homogalacturonan and arabinogalactan proteins in developing ovules of the gymnosperm plant Larix decidua Mill. Sex Plant Reprod 24:75–87
Renzaglia KS, Lopez RA, Johnson EE (2014) Callose is integral to the development of permanent tetrads in the liverwort Sphaerocarpos. Planta 241:615–627
Roberts S, Brownlee C (1995) Calcium influx, fertilisation potential and egg activation in Fucus serratus. Zygote 3:191–197
Runft LL, Jaffe LA, Mehlmann LM (2002) Egg activation at fertilization: where it all begins. Dev Biol 245:237–254
Smallwood M, Yates EA, Willats WG, Martin H, Knox JP (1996) Immunochemical comparison of membrane-associated and secreted arabinogalactan-proteins in rice and carrot. Planta 198:452–459
Sørensen I, Domozych D, Willats WG (2010) How have plant cell walls evolved? Plant Physiol 153:366–372
Southworth D, Kwiatkowski S (1996) Arabinogalactan proteins at the cell surface of Brassica sperm and Lilium sperm and generative cells. Sex Plant Reprod 9:269–272
Tang TS, Dong JB, Huang XY, Sun FZ (2000) Ca2+ oscillations induced by a cytosolic sperm protein factor are mediated by a maternal machinery that functions only once in mammalian eggs. Development 127:1141–1150
Tian HQ, Russell SD (1997) Calcium distribution in fertilized and unfertilized ovules and embryo sacs of Nicotiana tabacum L. Planta 202:93–105
Tian HQ, Zhu H, Russell SD (2000) Calcium changes in ovules and embryo sacs of Plumbago zeylanica L. Sex Plant Reprod 13:11–20
Ulvskov P, Wium H, Bruce D, Jørgensen B, Qvist KB, Skjøt M, Hepworth D, Borkhardt B, Sørensen SO (2005) Biophysical consequences of remodeling the neutral side chains of rhamnogalacturonan I in tubers of transgenic potatoes. Planta 220:609–620
Verhertbruggen Y, Knox JP (2007) Pectic polysaccharides and expanding cell walls. In: Verbelen J-P, Vissenberg K (eds) Plant cell monographs: the expanding cell, vol 5. Springer, Berlin, pp 139–158
Verhertbruggen Y, Marcus SE, Haeger A, Ordaz-Ortiz JJ, Knox JP (2009a) An extended set of monoclonal antibodies to pectic homogalacturonan. Carbohyd Res 344:1858–1862
Verhertbruggen YS, Marcus SE, Haeger A, Verhoef R, Schols HA, McCleary BV, McKee L, Gilbert HG, Knox JP (2009b) Developmental complexity of arabinan polysaccharides and their processing in plant cell walls. Plant J 59:413–425
Warne TR, Walker GL, Hickok LG (1986) A novel method for surface-sterilizing and sowing fern spores. Am Fern J 76:187–188
Watkins JE, Mack MC, Sinclair TR, Mulkey SS (2007) Ecological and evolutionary consequences of desiccation tolerance in tropical fern gametophytes. New Phytol 176:708–717
Willats WG, Marcus SE, Knox JP (1998) Generation of a monoclonal antibody specific to (1,5)-α-l-arabinan. Carbohyd Res 308:149–152
Willats WG, Steele-King CG, Marcus SE, Knox JP (1999) Side chains of pectic polysaccharides are regulated in relation to cell proliferation and cell differentiation. Plant J 20:619–628
Yariv J, Lis H, Katchalski E (1967) Precipitation of Arabic acid and some seed polysaccharides by glycosyl phenylazo dyes. Biochem J 105:1c–2c
Yates EA, Valdor J-F, Haslam SM, Morris HR, Dell A, Mackie W, Knox JP (1996) Characterization of carbohydrate structural features recognized by anti-arabinogalactan-protein monoclonal antibodies. Glycobiology 6:131–139
Yu F, Zhao J, Liang S, Yang H (1998) Ultracytochemical localization of calcium in the gynoecium and embryo sac of rice. Acta Bot Sin 41:125–129
Acknowledgments
We thank Les Hickok for the Ceratopteris spores used in this study. We also thank Bryan Piatkowski, Amelia Merced, Nicholas Flowers and Jason Henry for technical support and comments on the manuscript. This work was supported by the National Science Foundation (Grants DEB-0423625, DEB-0521177, DEB-0638722).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lopez, R.A., Renzaglia, K.S. Arabinogalactan proteins and arabinan pectins abound in the specialized matrices surrounding female gametes of the fern Ceratopteris richardii . Planta 243, 947–957 (2016). https://doi.org/10.1007/s00425-015-2448-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-015-2448-4