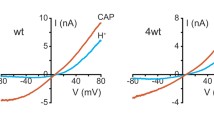
Abstract
Propofol, a commonly used intravenous anesthetic agent, is known to at times cause pain sensation upon injection in humans. However, the molecular mechanisms underlying this effect are not fully understood. Although propofol was reported to activate human transient receptor potential ankyrin 1 (TRPA1) in this regard, its action on human TRP vanilloid 1 (TRPV1), another nociceptive receptor, is unknown. Furthermore, whether propofol activates TRPV1 in rodents is controversial. Here, we show that propofol activates human and mouse TRPA1. In contrast, we did not observe propofol-evoked human TRPV1 activation, while the ability of propofol to activate mouse TRPV1 was very small. We also found that propofol caused increases in intracellular Ca2+ concentrations in a considerable portion of dorsal root ganglion (DRG) cells from mice lacking both TRPV1 and TRPA1, indicating the existence of TRPV1- and TRPA1-independent mechanisms for propofol action. In addition, propofol produced action potential generation in a type A γ-amino butyric acid (GABAA) receptor-dependent manner. Finally, we found that both T-type and L-type Ca2+ channels are activated downstream of GABAA receptor activation by propofol. Thus, we conclude that propofol may cause pain sensation through multiple mechanisms involving not only TRPV1 and TRPA1 but also voltage-gated channels downstream of GABAA receptor activation.





Similar content being viewed by others
References
Alkire MT, Hudetz AG, Tononi G (2008) Consciousness and anesthesia. Science 322:876–880. doi:10.1126/science.1149213
Aptel H, Hilaire C, Pieraut S, Boukhaddaoui H, Mallie S, Valmier J, Scamps F (2007) The Cav3.2/alpha1H T-type Ca2+ current is a molecular determinant of excitatory effects of GABA in adult sensory neurons. Mol Cell Neurosci 36:293–303. doi:10.1016/j.mcn.2007.07.009
Ault B, Hildebrand LM (1994) GABAA receptor-mediated excitation of nociceptive afferents in the rat isolated spinal cord-tail preparation. Neuropharmacology 33:109–114
Bautista DM, Jordt SE, Nikai T, Tsuruda PR, Read AJ, Poblete J, Yamoah EN, Basbaum AI, Julius D (2006) TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell 124:1269–1282. doi:10.1016/j.cell.2006.02.023
Cao E, Cordero-Morales JF, Liu B, Qin F, Julius D (2013) TRPV1 channels are intrinsically heat sensitive and negatively regulated by phosphoinositide lipids. Neuron 77:667–679. doi:10.1016/j.neuron.2012.12.016
Carlton SM, Zhou S, Coggeshall RE (1999) Peripheral GABAA receptors: evidence for peripheral primary afferent depolarization. Neuroscience 93:713–722
Caterina MJ, Leffler A, Malmberg AB, Martin WJ, Trafton J, Petersen-Zeitz KR, Koltzenburg M, Basbaum AI, Julius D (2000) Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science 288:306–313
Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D (1997) The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 389:816–824. doi:10.1038/39807
Chung MK, Jung SJ, Oh SB (2011) Role of TRP channels in pain sensation. Adv Exp Med Biol 704:615–636. doi:10.1007/978-94-007-0265-3_33
Coull JA, Boudreau D, Bachand K, Prescott SA, Nault F, Sik A, De Koninck P, De Koninck Y (2003) Trans-synaptic shift in anion gradient in spinal lamina I neurons as a mechanism of neuropathic pain. Nature 424:938–942. doi:10.1038/nature01868
Doenicke AW, Roizen MF, Rau J, Kellermann W, Babl J (1996) Reducing pain during propofol injection: the role of the solvent. Anesth Analg 82:472–474
Dundee JW (1979) New i.v. anaesthetics. Br J Anaesth 51:641–648
Fischer MJ, Leffler A, Niedermirtl F, Kistner K, Eberhardt M, Reeh PW, Nau C (2010) The general anesthetic propofol excites nociceptors by activating TRPV1 and TRPA1 rather than GABAA receptors. J Biol Chem 285:34781–34792. doi:10.1074/jbc.M110.143958
Franks NP (2006) Molecular targets underlying general anaesthesia. Br J Pharmacol 147(Suppl 1):S72–S81. doi:10.1038/sj.bjp.0706441
Funk K, Woitecki A, Franjic-Wurtz C, Gensch T, Mohrlen F, Frings S (2008) Modulation of chloride homeostasis by inflammatory mediators in dorsal root ganglion neurons. Mol Pain 4:32. doi:10.1186/1744-8069-4-32
Garcia PS, Kolesky SE, Jenkins A (2010) General anesthetic actions on GABAA receptors. Curr Neuropharmacol 8:2–9. doi:10.2174/157015910790909502
Hinman A, Chuang HH, Bautista DM, Julius D (2006) TRP channel activation by reversible covalent modification. Proc Natl Acad Sci U S A 103:19564–19568. doi:10.1073/pnas.0609598103
Jalota L, Kalira V, George E, Shi YY, Hornuss C, Radke O, Pace NL, Apfel CC (2011) Prevention of pain on injection of propofol: systematic review and meta-analysis. BMJ 342:d1110. doi:10.1136/bmj.d1110
Jurd R, Arras M, Lambert S, Drexler B, Siegwart R, Crestani F, Zaugg M, Vogt KE, Ledermann B, Antkowiak B, Rudolph U (2003) General anesthetic actions in vivo strongly attenuated by a point mutation in the GABAA receptor beta3 subunit. FASEB J 17:250–252. doi:10.1096/fj.02-0611fje
Kakazu Y, Akaike N, Komiyama S, Nabekura J (1999) Regulation of intracellular chloride by cotransporters in developing lateral superior olive neurons. J Neurosci 19:2843–2851
Klein RM, Ufret-Vincenty CA, Hua L, Gordon SE (2008) Determinants of molecular specificity in phosphoinositide regulation. Phosphatidylinositol (4,5)-bisphosphate (PI(4,5)P2) is the endogenous lipid regulating TRPV1. J Biol Chem 283:26208–26216. doi:10.1074/jbc.M801912200
Klement W, Arndt JO (1991) Pain on injection of propofol: effects of concentration and diluent. Br J Anaesth 67:281–284
Kurganov E, Zhou Y, Saito S, Tominaga M (2014) Heat and AITC activate green anole TRPA1 in a membrane-delimited manner. Pflugers Arch 466:1873–1884. doi:10.1007/s00424-013-1420-z
Lee SP, Buber MT, Yang Q, Cerne R, Cortes RY, Sprous DG, Bryant RW (2008) Thymol and related alkyl phenols activate the hTRPA1 channel. Br J Pharmacol 153:1739–1749. doi:10.1038/bjp.2008.85
Macpherson LJ, Dubin AE, Evans MJ, Marr F, Schultz PG, Cravatt BF, Patapoutian A (2007) Noxious compounds activate TRPA1 ion channels through covalent modification of cysteines. Nature 445:541–545. doi:10.1038/nature05544
Mandadi S, Tominaga T, Numazaki M, Murayama N, Saito N, Armati PJ, Roufogalis BD, Tominaga M (2006) Increased sensitivity of desensitized TRPV1 by PMA occurs through PKCepsilon-mediated phosphorylation at S800. Pain 123:106–116. doi:10.1016/j.pain.2006.02.016
Mao S, Garzon-Muvdi T, Di Fulvio M, Chen Y, Delpire E, Alvarez FJ, Alvarez-Leefmans FJ (2012) Molecular and functional expression of cation-chloride cotransporters in dorsal root ganglion neurons during postnatal maturation. J Neurophysiol 108:834–852. doi:10.1152/jn.00970.2011
Matta JA, Cornett PM, Miyares RL, Abe K, Sahibzada N, Ahern GP (2008) General anesthetics activate a nociceptive ion channel to enhance pain and inflammation. Proc Natl Acad Sci U S A 105:8784–8789. doi:10.1073/pnas.0711038105
Mohammadi B, Haeseler G, Leuwer M, Dengler R, Krampfl K, Bufler J (2001) Structural requirements of phenol derivatives for direct activation of chloride currents via GABAA receptors. Eur J Pharmacol 421:85–91
Moriyama T, Iida T, Kobayashi K, Higashi T, Fukuoka T, Tsumura H, Leon C, Suzuki N, Inoue K, Gachet C, Noguchi K, Tominaga M (2003) Possible involvement of P2Y2 metabotropic receptors in ATP-induced transient receptor potential vanilloid receptor 1-mediated thermal hypersensitivity. J Neurosci 23:6058–6062
Numazaki M, Tominaga T, Takeuchi K, Murayama N, Toyooka H, Tominaga M (2003) Structural determinant of TRPV1 desensitization interacts with calmodulin. Proc Natl Acad Sci U S A 100:8002–8006. doi:10.1073/pnas.1337252100
Numazaki M, Tominaga T, Toyooka H, Tominaga M (2002) Direct phosphorylation of capsaicin receptor VR1 by protein kinase C epsilon and identification of two target serine residues. J Biol Chem 277:13375–13378. doi:10.1074/jbc.C200104200
Orser BA, Wang LY, Pennefather PS, MacDonald JF (1994) Propofol modulates activation and desensitization of GABAA receptors in cultured murine hippocampal neurons. J Neurosci 14:7747–7760
Rosenbaum T, Gordon-Shaag A, Munari M, Gordon SE (2004) Ca2+/calmodulin modulates TRPV1 activation by capsaicin. J Gen Physiol 123:53–62. doi:10.1085/jgp.200308906
Rudolph U, Antkowiak B (2004) Molecular and neuronal substrates for general anaesthetics. Nat Rev Neurosci 5:709–720. doi:10.1038/nrn1496
Scroggs RS, Fox AP (1992) Calcium current variation between acutely isolated adult rat dorsal root ganglion neurons of different size. J Physiol 445:639–658
Stein V, Nicoll RA (2003) GABA generates excitement. Neuron 37:375–378
Stein AT, Ufret-Vincenty CA, Hua L, Santana LF, Gordon SE (2006) Phosphoinositide 3-kinase binds to TRPV1 and mediates NGF-stimulated TRPV1 trafficking to the plasma membrane. J Gen Physiol 128:509–522. doi:10.1085/jgp.200609576
Story GM, Peier AM, Reeve AJ, Eid SR, Mosbacher J, Hricik TR, Earley TJ, Hergarden AC, Andersson DA, Hwang SW, McIntyre P, Jegla T, Bevan S, Patapoutian A (2003) ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell 112:819–829
Tan CH, Onsiong MK (1998) Pain on injection of propofol. Anaesthesia 53:468–476. doi:10.1046/j.1365-2044.1998.00405.x
Tominaga M, Caterina MJ, Malmberg AB, Rosen TA, Gilbert H, Skinner K, Raumann BE, Basbaum AI, Julius D (1998) The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron 21:531–543
Usala M, Thompson SA, Whiting PJ, Wafford KA (2003) Activity of chlormethiazole at human recombinant GABAA and NMDA receptors. Br J Pharmacol 140:1045–1050. doi:10.1038/sj.bjp.0705540
Wang YY, Chang RB, Waters HN, McKemy DD, Liman ER (2008) The nociceptor ion channel TRPA1 is potentiated and inactivated by permeating calcium ions. J Biol Chem 283:32691–32703. doi:10.1074/jbc.M803568200
Yevenes GE, Zeilhofer HU (2011) Allosteric modulation of glycine receptors. Br J Pharmacol 164:224–236. doi:10.1111/j.1476-5381.2011.01471.x
Zeller A, Arras M, Lazaris A, Jurd R, Rudolph U (2005) Distinct molecular targets for the central respiratory and cardiac actions of the general anesthetics etomidate and propofol. FASEB J 19:1677–1679. doi:10.1096/fj.04-3443fje
Zurborg S, Yurgionas B, Jira JA, Caspani O, Heppenstall PA (2007) Direct activation of the ion channel TRPA1 by Ca2+. Nat Neurosci 10:277–279. doi:10.1038/nn1843
Acknowledgments
We would like to thank Dr. Junichi Nabekura (Division of Homeostatic Development, National Institute for Physiological Sciences, Okazaki, Japan), Dr. Motohiro Nishida (Division of Cardiocirculatory Signaling, National Institute for Physiological Sciences, Okazaki, Japan), Dr. Hidemasa Furue (Division of Neural Signaling, National Institute for Physiological Sciences, Okazaki, Japan), and Dr. Hitoshi Ishibashi (School of Allied Health Sciences, Kitasato University, Kanagawa, Japan) for their helpful suggestion and discussion. This work was supported by grants to Makoto Tominaga from the Ministry of Education, Culture, Sports, Science and Technology in Japan.
Conflict of interest
The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nishimoto, R., Kashio, M. & Tominaga, M. Propofol-induced pain sensation involves multiple mechanisms in sensory neurons. Pflugers Arch - Eur J Physiol 467, 2011–2020 (2015). https://doi.org/10.1007/s00424-014-1620-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-014-1620-1