Abstract
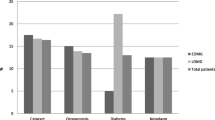
Leflunomide is an effective drug used in the treatment of rheumatoid arthritis. Here we report the findings of an open-label pilot study, which found that leflunomide is also an effective treatment for myasthenia gravis (MG). This study recruited 15 corticosteroid-dependent MG patients. For 6 months, leflunomide 20 mg was given to these patients daily along with prednisone. The quantitative myasthenia gravis (QMG) scores and MG activities of daily living (MG-ADL) profiles were measured in these MG patients. After 6 months of treatment, 9 of the 15 patients enrolled in this study showed improvements in both QMG and MG-ADL. The mean QMG scores (13.4 to 8.5) and MG-ADL profiles (5.8 to 2.8) were significantly decreased (P = 0.01, 0.006 respectively). Furthermore, we found that the mean corticosteroid doses were reduced after treatment with leflunomide (24.3 to 12.3 mg per day). Leflunomide is a well-tolerated and efficacious treatment for corticosteroid-dependent MG, which may also enable lower doses of corticosteroids to be administered.

Similar content being viewed by others
References
Rowin J, Meriggioli MN, Tuzun E, Leurgans S, Christadoss P (2004) Etanercept treatment in corticosteroid-dependent myasthenia gravis. Neurology 63:2390–2392
Ponseti JM, Azem J, Fort JM, Lopez-Cano M, Vilallonga R, Buera M, Cervera C, Armengol M (2005) Long-term results of tacrolimus in cyclosporine- and prednisone-dependent myasthenia gravis. Neurology 64:1641–1643
Kawaguchi N, Yoshiyama Y, Nemoto Y, Munakata S, Fukutake T, Hattori T (2004) Low-dose tacrolimus treatment in thymectomised and steroid-dependent myasthenia gravis. Curr Med Res Opin 20:1269–1273
Nagaishi A, Yukitake M, Kuroda Y (2008) Long-term treatment of steroid-dependent myasthenia gravis patients with low-dose tacrolimus. Intern Med 47:731–736
Griffin R, Levine T, Mozaffar T, Nowak R, Pulley M, Dimachkie M, Nicolle M (2015) Efficacy and safety of IGIV-C in corticosteroid dependent patients with generalized myasthenia gravis. https://www.clinicaltrials.gov/ct2/show/NCT02473965
Tindall RS, Rollins JA, Phillips JT, Greenlee RG, Wells L, Belendiuk G (1987) Preliminary results of a double-blind, randomized, placebo-controlled trial of cyclosporine in myasthenia gravis. N Engl J Med 316:719–724
Palace J, Newsom-Davis J, Lecky B (1998) A randomized double-blind trial of prednisolone alone or with azathioprine in myasthenia gravis. Myasthenia Gravis Study Group. Neurology 50:1778–1783
Sanders DB, Evoli A (2010) Immunosuppressive therapies in myasthenia gravis. Autoimmunity 43:428–435
Meriggioli MN, Sanders DB (2009) Autoimmune myasthenia gravis: emerging clinical and biological heterogeneity. Lancet Neurol 8:475–490
Gilhus NE, Verschuuren JJ (2015) Myasthenia gravis: subgroup classification and therapeutic strategies. Lancet Neurol 14:1023–1036
Cherwinski HM, Byars N, Ballaron SJ, Nakano GM, Young JM, Ransom JT (1995) Leflunomide interferes with pyrimidine nucleotide biosynthesis. Inflamm Res 44:317–322
O’Connor P, Wolinsky JS, Confavreux C, Comi G, Kappos L, Olsson TP, Benzerdjeb H, Truffinet P, Wang L, Miller A, Freedman MS (2011) Randomized trial of oral teriflunomide for relapsing multiple sclerosis. N Engl J Med 365:1293–1303
Confavreux C, O’Connor P, Comi G, Freedman MS, Miller AE, Olsson TP, Wolinsky JS, Bagulho T, Delhay JL, Dukovic D, Truffinet P, Kappos L (2014) Oral teriflunomide for patients with relapsing multiple sclerosis (TOWER): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Neurol 13:247–256
O’Connor PW, Li D, Freedman MS, Bar-Or A, Rice GP, Confavreux C, Paty DW, Stewart JA, Scheyer R (2006) A phase II study of the safety and efficacy of teriflunomide in multiple sclerosis with relapses. Neurology 66:894–900
Vidic-Dankovic B, Kosec D, Damjanovic M, Apostolski S, Isakovic K, Bartlett RR (1995) Leflunomide prevents the development of experimentally induced myasthenia gravis. Int J Immunopharmacol 17(4):273–281
Skeie GO, Apostolski S, Evoli A, Gilhus NE, Illa I, Harms L, Hilton-Jones D, Melms A, Verschuuren J, Horge HW, European Federation of Neurological S (2010) Guidelines for treatment of autoimmune neuromuscular transmission disorders. Eur J Neurol 17:893–902
Leite MI, Jacob S, Viegas S, Cossins J, Clover L, Morgan BP, Beeson D, Willcox N, Vincent A (2008) IgG1 antibodies to acetylcholine receptors in ‘seronegative’ myasthenia gravis. Brain 131:1940–1952
Cossins J, Belaya K, Zoltowska K, Koneczny I, Maxwell S, Jacobson L, Leite MI, Waters P, Vincent A, Beeson D (2012) The search for new antigenic targets in myasthenia gravis. Ann N Y Acad Sci 1275:123–128
Burns TM (2010) History of outcome measures for myasthenia gravis. Muscle Nerve 42:5–13
Ciafaloni E, Massey JM, Tucker-Lipscomb B, Sanders DB (2001) Mycophenolate mofetil for myasthenia gravis: an open-label pilot study. Neurology 56:97–99
Poor G, Strand V, Leflunomide Multinational Study G (2004) Efficacy and safety of leflunomide 10 mg versus 20 mg once daily in patients with active rheumatoid arthritis: multinational double-blind, randomized trial. Rheumatol (Oxford) 43:744–749
van Riel PL, Smolen JS, Emery P, Kalden JR, Dougados M, Strand CV, Breedveld FC (2004) Leflunomide: a manageable safety profile. J Rheumatol Suppl 71:21–24
Alcorn N, Saunders S, Madhok R (2009) Benefit-risk assessment of leflunomide: an appraisal of leflunomide in rheumatoid arthritis 10 years after licensing. Drug Saf 32:1123–1134
Keen HI, Conaghan PG, Tett SE (2013) Safety evaluation of leflunomide in rheumatoid arthritis. Expert Opin Drug Saf 12:581–588
Sanders DB, Hart IK, Mantegazza R, Shukla SS, Siddiqi ZA, De Baets MH, Melms A, Nicolle MW, Solomons N, Richman DP (2008) An international, phase III, randomized trial of mycophenolate mofetil in myasthenia gravis. Neurology 71:400–406
The Muscle Study Group (2008) A trial of mycophenolate mofetil with prednisone as initial immunotherapy in myasthenia gravis. Neurology 71:394–399
Sanders DB, Siddiqi ZA (2008) Lessons from two trials of mycophenolate mofetil in myasthenia gravis. Ann N Y Acad Sci 1132:249–253
Acknowledgments
This work was funded by the China National Natural Science Foundation (30870850, 81071002 and 81371386) and the Clinic Study of 5010 Plan, Sun Yat-sen University (2010003).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Ethical standards
This study was approved by the appropriate ethics committees in the First Affiliated Hospital of Sun Yat-sen University and complied with the standards set by the 1964 Declaration of Helsinki, and its later amendments. All participants gave informed consent prior to enrollment in the study.
Rights and permissions
About this article
Cite this article
Chen, P., Feng, H., Deng, J. et al. Leflunomide treatment in corticosteroid-dependent myasthenia gravis: an open-label pilot study. J Neurol 263, 83–88 (2016). https://doi.org/10.1007/s00415-015-7944-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-015-7944-8