Abstract
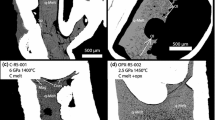
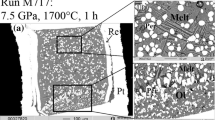
Experiments are applied to constrain the composition of primary kimberlitic magmas which were in equilibrium with lithospheric peridotite and could resorb the entrained diamond to form typical dissolution features. The experiments are run on samples of a model carbonatite and a melt of the Udachnaya kimberlite at 6.3 GPa and 1400 °C, and at unbuffered or Re–ReO2-buffered oxygen fugacity (1–2 log units above Ni–NiO). Near-liquidus dry Fe3+-free carbonatitic melt (derived from carbonated harzburgite) is saturated with the Ol–Grt–Opx–Mgs assemblage and is almost inert to diamond. Carbonatitic melts that bear 4.6–6.8 wt% Fe2O3 or 1.5 wt% H2O are in equilibrium only with Mgs ± Ol near the liquidus. Dissolution of diamond by these melts produces surface textures uncommon (corrosion sculptures) or common (negative-oriented trigons, shield-shaped laminae and elongate hillocks) to kimberlitic diamonds. The near-liquidus melt of the Udachnaya kimberlite (Yakutia) with 10–12 wt% H2O is saturated with the Ol–Grt–Cpx assemblage and may result from melting of carbonated garnet-bearing wehrlite. Hydrous kimberlitic melt likewise resorbs diamonds forming typical negative-oriented trigons, shield-shaped laminae and elongate hillocks on their surfaces. Therefore, the melts that could originate in the thermal conditions of subcratonic lithosphere, entrain diamond and dissolve it to produce dissolution features on crystal surfaces, were compositionally close to kimberlite (16–19 wt% SiO2) and rich in H2O. Dry Fe3+-bearing carbonatites with fO2 controlled by the ferric/ferrous equilibrium slightly above the Ni–NiO buffer cannot be diamond carriers.











Similar content being viewed by others
References
Arculus RJ, Dawson JB, Mitchell RH, Gust DA, Holmes RD (1984) Oxidation states of the upper mantle recorded by megacryst ilmenite in kimberlite and type A and B spinel lherzolites. Contrib Mineral Petrol 85:85–94
Arima M, Nakayama K, Akaishi M, Yamaoka S, Kanda H (1993) Crystallization of diamond from a silicate melt of kimberlite composition in high-pressure and high-temperature experiment. Geology 21:968–970
Bataleva YuV, Palyanov YN, Sokol AG, Borzdov YuM, Palyanova GA (2012) Conditions for the origin of oxidized carbonate–silicate melts: implications for mantle metasomatism and diamond formation. Lithos 128–131:113–125
Becker M, le Roex AP (2006) Geochemistry of South African on-and off-craton, Group I and Group II kimberlites: petrogenesis and source region evolution. J Petrol 47(4):673–703
Boettcher AL, Mysen BO, Allen JC (1973) Techniques for the control of water fugacity and oxygen fugacity for experimentation in solid-media high-pressure apparatus. J Geophys Res 78(26):5898–5901
Boyd FR, Pokhilenko NP, Pearson DG, Mertzman SA, Sobolev NV, Finger LW (1997) Composition of the Siberian cratonic mantle: evidence from Udachnaya peridotite xenoliths. Contrib Mineral Petrol 128:228–246
Brey GP, Bulatov VK, Girnis AV, Lahaye Y (2008) Experimental melting of carbonated peridotite at 6–10 GPa. J Petrol 49(4):797–821
Brey GP, Bulatov VK, Girnis AV (2009) Influence of water and fluorine on melting of carbonated peridotite at 6 and 10 GPa. Lithos 112:249–259
Brey GP, Bulatov VK, Girnis AV (2011) Melting of K-rich carbonated peridotite at 6–10 GPa and the stability of K-phases in the upper mantle. Chem Geol 281:333–342
Canil D, Fedortchouk Y (1999) Garnet dissolution and the emplacement of kimberlites. Earth Plan Sci Lett 167:227–237
Creighton S, Stachel T, Matveev S, Hofer H, McCammon C, Luth RW (2009) Oxidation of the Kaapvaal lithospheric mantle driven by metasomatism. Contrib Mineral Petrol 157:491–504
Dalton JA, Presnall DC (1998) The continuum of primary carbonatitic–kimberlitic melt compositions in equilibrium with lherzolite: data from the system CaO–MgO–Al2O3–SiO2–CO2 at 6 GPa. J Petrol 39(11-12):1953–1964
Dasgupta R, Hirschmann MM (2007) Effect of variable carbonate concentration on the solidus of mantle peridotite. Am Mineral 92:370–379
Dasgupta R, Mallik A, Tsuno K et al (2013) Carbon-dioxide-rich silicate melt in the Earth’s upper mantle. Nature 493:211–222
Davis RM, O’Relly SY, Griffin WL (1999) Diamonds from Wellington, NSW: insights into the origin of eastern Australian diamonds. Mineral Mag 63:447–471
Demouchy S, Jacobsen SD, Gaillard F, Stern CR (2006) Rapid magma ascent recorded by water diffusion profiles in mantle olivine. Geology 34:429–432
Edgar AD, Charbonneau HE (1993) Melting experiments on a SiO2-poor, CaO-rich aphanitic kimberlite from 5–10 GPa and their bearing on sources of kimberlite magmas. Am Mineral 78:132–142
Edgar AD, Arima M, Baldwin DK et al (1998) High-pressure high-temperature melting experiments on a SiO2-poor aphanitic kimberlite from the Wesselton mine, Kimberley, South Africa. Am Mineral 73:524–533
Eggler DH, Wendlandt RF (1979) Experimental studies on the relationships between kimberlite magma and partial melting of peridotite. In: Boyd FR, Meyer HOA (eds) Kimberlites, diatremes and diamonds: their geology, petrology, and geochemistry. American Geophysical Union, Washington, pp 331–378
Fedortchouk Y, Canil D (2004) Intensive variables in kimberlite magmas, Lac de Gras, Canada and implications for diamond survival. J Petrol 45:1725–1745
Fedortchouk Y, Canil D, Carlson JA (2005) Dissolution form in Las de Gras diamond and their relationship to the temperature and redox state of kimberline magma. Contrib Mineral Petrol 150:54–69
Fedortchouk Y, Canil D, Semenets E (2007) Mechanisms of diamond oxidation and their bearing on the fluid composition in kimberlite magmas. Am Mineral 92:1200–1212
Foley SF (2011) A reappraisal of redox melting in the Earth’s mantle as a function of tectonic setting and time. J Petrol 52:1363–1391
Foley SF, Yaxley GM, Rosenthal A, Buhre S, Kiseeva ES, Rapp RP, Jacob DE (2009) The composition of near-solidus melts of peridotite in the presence of CO2 and H2O between 40 and 60 kbar. Lithos 112:274–283
Franz L, Brey GP, Okrusch M (1996) Reequilibration of ultramafic xenoliths from Namibia by metasomatic processes at the mantle boundary. J Geol 104:599–615
Girnis AV, Brey GP, Ryabchikov ID (1995) Origin of Group IA kimberlites: fluid saturated melting experiments at 45–55 kbar. Earth Planet Sci Lett 134:283–296
Girnis AV, Bulatov VK, Brey GP (2011) Formation of primary kimberlite melts—constraints from experiments at 6–12 GPa and variable CO2/H2O. Lithos 127:401–413
Goncharov AG, Ionov DA, Doucet LS, Pokhilenko LN (2012) Thermal state, oxygen fugacity and C-O-H fluid speciation in cratonic lithospheric mantle: new data on peridotite xenoliths from the Udachnaya kimberlite, Siberia. Earth Planet Sci Lett 357:99–110
Harris M, le Roex A, Class C (2004) Geochemistry of the Uintjiesberg kimberlite, South Africa: petrogenesis of an off-craton, group I, kimberlite. Lithos 74:149–165
Höfer HE, Lazarov M, Brey GP, Woodland AB (2009) Oxygen fugacity of the metasomatizing melt in a polymict peridotite from Kimberley. Lithos 112:1150–1154
Howarth GH, Barry PH, Pernet-Fisher JF, Baziotis IP, Pokhilenko NP, Pokhilenko LN, Bodnar RJ, Taylor LA, Agashev AM (2014) Superplume metasomatism: evidence from Siberian mantle xenoliths. Lithos 184–187:209–224
Kamenetsky VS, Kamenetsky MB, Weiss Y, Navon O et al (2009) How unique is the Udachnaya-east kimberlite: comparison with kimberlites from the Slave Craton (Canada) and SW Greenland. Lithos 112:334–346
Kamenetsky VS, Kamenetsky MB, Golovin AV, Sharygin VV, Maas R (2012) Ultrafresh salty kimberlite of the Udachnaya_East pipe (Yakutiya, Russia): a petrological oddity or fortuitous discovery? Lithos 152:173–186
Kanda H, Yamaoka S, Setaka N, Komatsu H (1977) Etching of diamond octahedrons by high pressure water. J Cryst Growth 38:1–7
Kelley SP, Wartho JA (2000) Rapid kimberlite ascent and the significance of Ar–Ar ages in xenolith phlogopites. Science 289:609–611
Kennedy CS, Kennedy G (1976) The equilibrium boundary between graphite and diamond. J Geophys Res 81:2467–2470
Khokhryakov AF, Palyanov YN (2007) The evolution of diamond morphology in the process of dissolution: experimental data. Am Mineral 92:909–917
Khokhryakov AF, Palyanov YN (2010) Influence of the fluid composition on diamond dissolution forms in carbonate melts. Am Mineral 95:1508–1514
Khokhryakov AF, Palyanov YN (2015) Effect of crystal defects on diamond morphology during dissolution in the mantle. Am Mineral 100:1528–1532
Kjarsgaard BA, Pearson DG, Tappe S, Nowell GM, Dowall DP (2009) Geochemistry of hypabyssal kimberlites from Lac de Gras, Canada: comparisons to a global database and applications to the parent magma problem. Lithos 112:236–248
Klein-BenDavid O, Izraeli ES, Hauri E, Navon O (2007) Fluid inclusions in diamonds from the Diavik mine, Canada and the evolution of diamond-forming fluids. Geochim Cosmochim Acta 71(3):723–744
Klein-BenDavid O, Logvinova AM, Schrauder M et al (2009) High-Mg carbonatitic microinclusions in some Yakutian diamonds a new type of diamond-forming fluid. Lithos 112S:648–659
Kopylova MG, Matveev S, Raudsepp M (2007) Searching for parental kimberlite melt. Geochim Cosmochim Acta 71:3616–3629
Kozai Y, Arima M (2005) Experimental study on diamond dissolution in kimberlitic and lamproitic melts at 1300–1420 °C and 1 GPa with controlled oxygen partial pressure. Am Mineral 90:1759–1766
le Roex AP, Bell DR, Davis P (2003) Petrogenesis of group I kimberlites from Kimberley, South Africa: evidence from bulk-rock geochemistry. J Petrol 44:2261–2286
Luth RW (1989) Natural versus experimental control of oxidation state: effects on the composition and speciation of C–O–H fluids. Am Mineral 74:50–57
Meyer HO (1985) Genesis of diamond: a mantle saga. Am Mineral 70:344–355
Mitchell RH (1973) Composition of olivine, silica activity and oxygen fugacity in kimberlite. Lithos 6:65–81
Mitchell RH (1986) Kimberlites: mineralogy, geochemistry and petrology. Plenum Press, New York
Mitchell RH (2004) Experimental studies at 5–12 GPa of the Ondermatjie hypabyssal kimberlite. Lithos 76:551–564
Mitchell RH (2008) Petrology of hypabyssal kimberlites: relevance to primary magma compositions. J Volcanol Geotherm Res 174:1–8
Mitchell R, Tappe S (2010) Discussion of “Kimberlites and aillikites as probes of the continental lithospheric mantle”, by D. Francis and M. Patterson (Lithos v. 109, p. 72–80). Lithos 115:288–292
Navon O (1999) Diamond formation in the Earth’s mantle. In: Gurney JJ, Gurney JL, Pascoe MD, Richadson SH (eds) VII International Kimberlite conference 2. Cape Town: Red roof design, pp 584–604
Nechaev DV, Khokhryakov AF (2013) Formation of epigenetic graphite inclusions in diamond crystals: experimental data. Russ Geol Geophys 54:399–405
Orlov IL (1977) The mineralogy of the diamond. Wiley, New York
Pal’yanov YN, Sokol AG, Borzdov YM, Khokhryakov AF, Sobolev NV (1999) Diamond formation from mantle carbonate fluids. Nature 400:417–418
Pal’yanov YN, Sokol AG, Borzdov YM, Khokhryakov AF (2002) Fluid-bearing alkaline–carbonate melts as the medium for the formation of diamonds in the Earth’s mantle: an experimental study. Lithos 60:145–159
Pal’yanov YN, Sokol AG, Tomilenko AA, Sobolev NV (2005) Conditions of diamond formation through carbonate–silicate interaction. Eur J Mineral 17:207–214
Palyanov YN, Sokol AG (2009) The effect of composition of mantle fluid/melts on diamond formation processes. Lithos 112S:690–700
Palyanov YN, Borzdov YuM, Khokhryakov AF, Kupriyanov IN, Sokol AG (2010) Effect of nitrogen impurity on diamond crystal growth processes. Cryst Growth Des 10:3169–3175
Palyanov YN, Sokol AG, Khokhryakov AF, Kruk AN (2015) Conditions of diamond crystallization in kimberlite melt: experimental data. Russ Geol Geophys 56:196–210
Pearson DG, Kelley SP, Pokhilenko NP, Boyd FR (1997) Laser 40Ar/39Ar dating of phlogopites from Southern African and Siberian kimberlites and their xenoliths: constraints on eruption ages, melt degassing and mantle volatile compositions. Geol Geofiz 38:106–117
Persikov ES (1998) Viscosities of model and magmatic melts at the TP-parameters of the Earth’s crust and upper mantle. Geol Geofiz 39:1780–1792
Pollack HN, Chapman DS (1977) On the regional variation of heat flow, geotherms, and lithospheric thickness. Tectonophysics 38:279–296
Pownceby MI, O’Neill HSC (1994) Thermodynamic data from redox reactions at high temperatures. IV. Calibration of the Re–ReO2 oxygen buffer from EMF and NiO + Ni-Pd redox sensor measurements. Contrib Mineral Petrol 118:130–137
Robinson DN, Scott JA, Niekerk AV, Anderson VG (1989) The sequence of events reflected in the diamonds of some southern African kimberlites. In Ross J (ed) Kimberlite and related rocks, vol. 2. Geological Society of Australia. Special Publication, Blackwell, Carlton, 14, pp 1042–1053
Rohrbach A, Schmidt MW (2011) Redox freezing and melting in the Earth’s deep mantle resulting from carbon-iron redox coupling. Nature 472:209–212
Rudenko AP, Kulakova II, Sturman VL (1979) Oxidation of natural diamond. Novye dannye o mineralogii SSSR. Academiya Nauk SSSR, Fersman Mineralogical Museum, Moscow, pp 105–125 (in Russian)
Sokol AG, Pal’yanov YN (2008) Diamond formation in the system MgO–SiO2–H2O–C at 7.5 GPa and 1600°C. Contrib Miner Petrol 155:33–43
Sokol AG, Pal’yanov YN, Pal’yanova GA, Tomilenko AA (2004) Diamond crystallization in fluid and carbonate-fluid systems under mantle P–T conditions: 1 Fluid composition. Geochem Int 42:830–838
Sokol AG, Kupriyanov IN, Palyanov YN, Kruk AN, Sobolev NV (2013a) Melting experiments on the Udachnaya kimberlite at 6.3–7.5 GPa: implications for the role of H2O in magma generation and formation of hydrous olivine. Geochim Cosmochim Acta 101:133–155
Sokol AG, Kupriyanov IN, Palyanov YN (2013b) Partitioning of H2O between olivine and carbonate–silicate melts at 6.3 GPa and 1400 °C: implications for kimberlite formation. Earth Planet Sci Lett 383:58–67
Sokol AG, Kruk AN, Palyanov YN (2014) The role of water in generation of group II kimberlite magmas: constraints from multiple saturation experiments. Am Mineral 99:2292–2302
Sokol AG, Borzdov YuM, Palyanov YN, Khokhryakov AF (2015) High-temperature calibration of a multi-anvil high-pressure apparatus. High Press Res 35:139–147
Sparks RSJ, Brooker RA, Field M, Kavanagh J, Schumacher JC, Walter MJ, White J (2009) The nature of erupting kimberlite melts. Lithos 112:429–438
Spera FJ (1984) Carbon dioxide in petrogenesis III: role of volatiles in the ascent of alkaline magma with special reference to xenolith-bearing mafic lavas. Contrib Mineral Petrol 88:217–232
Stagno V, Frost DJ (2010) Carbon speciation in the asthenosphere: experimental measurements of the redox conditions at which carbonate-bearing melts coexist with graphite or diamond in peridotite assemblages. Earth Planet Sci Lett 300:72–84
Stagno V, Ojwang DO, McCammon CA, Frost DJ (2013) The oxidation state of the mantle and the extraction of carbon from Earth’s interior. Nature 493(7430):84–88
Ulmer P, Sweeney RJ (2002) Generation and differentiation of group II kimberlites: constraints from a high-pressure experimental study to 10 GPa. Geochim Cosmochim Acta 66:2139–2153
Whitney JA (1972) The effect of reduced H2O fugacity on the buffering of oxygen fugacity in hydrothermal experiments. Am Mineral 57:1902–1908
Yamaoka S, Kanda H, Setaka N (1980) Etching of diamond octahedrons at high temperatures and pressures with controlled oxygen partial pressure. J Mater Sci 15:332–336
Acknowledgments
We wish to thank Yury Borzdov, Galina Palyanova and Alexey Kruk for their assistance throughout the study. The manuscript profited much from editorial handling and comments by Max Schmidt, as well as from thoughtful reviews by Andrei Girnis and the anonymous reviewer. The research was performed by Grant of the Russian Science Foundation (Project 14-27-00054).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Max W. Schmidt.
Rights and permissions
About this article
Cite this article
Sokol, A.G., Khokhryakov, A.F. & Palyanov, Y.N. Composition of primary kimberlite magma: constraints from melting and diamond dissolution experiments. Contrib Mineral Petrol 170, 26 (2015). https://doi.org/10.1007/s00410-015-1182-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00410-015-1182-z