Abstract
Purpose
In this study, we focused on the effect of hyperglycemia on the generation of reactive oxygen species and on the release of pro-inflammatory cytokines in the human monocytic cell line (U937). We also monitored potential anti-inflammatory effects of walnut oil as well as its protective effect against oxidative damage to biopolymers (DNA and proteins).
Methods
We cultured U937 cells under normoglycemic or hyperglycemic conditions for 72 h, in the absence or presence of walnut oil. We detected cell proliferation by the MTT test. To determine the antioxidant status of cells, we used the trolox equivalent antioxidant capacity method. We determined the activity of superoxide dismutase (SOD) spectrophotometrically, the oxidative damage to DNA by an enzyme-modified comet assay, and the oxidative damage to proteins by the marker—protein carbonyls and the levels of pro-inflammatory cytokines by the ELISA method.
Results
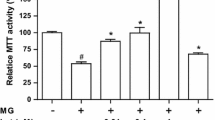
Hyperglycemia reduced the antioxidant capacity of cells, induced oxidative damage to DNA, and increased the release of pro-inflammatory cytokines. It had no effect on cell proliferation, SOD activity, nor oxidative damage to proteins. Walnut oil significantly increased the antioxidant capacity of cells as well as SOD activity on the second and third day of incubation, but had no effect on cell proliferation and showed no protective effect against oxidative damage to DNA and proteins. The walnut oil showed both anti-inflammatory and pro-inflammatory properties depending on its concentration and time of its incubation with the monocytic cell line.
Conclusion
Our in vitro results indicate that walnut oil can diminish oxidative stress with its antioxidant properties. However, we could not confirm its protective effect against oxidative damage to DNA and proteins.









Similar content being viewed by others
References
Chen F, Qian LH, Deng B (2013) Resveratrol protects vascular endothelial cells from high glucose-induced apoptosis through inhibition NADPH oxidase activation-driven oxidative stress. CNS Neurosci Ther 19:675–681
Matkins AC, Sanders RA, Watkins JB (2003) Diabetes, oxidative stress, and antioxidants: a review. J Biochem Mol Toxicol 17:24–38
Hadi HA, Carr CS, Al Suwaidi J (2005) Endothelial dysfunction: cardiovascular risk factors, therapy, and outcome. Vasc Health Risk Manag 1:183–193
Tousoulis D, Kampoli AM, Papageorgious N et al (2011) Pathophysiology of atherosclerosis: the role of inflammation. Curr Pharm Des 17:4089–4110
Tsai EC, Hirch IB, Brunzell JD, Chait A (1994) Reduced plasma peroxyl radical trapping capacity and increased susceptibility of LDL to oxidation in poorly controlled IDDM. Diabetes 43:1010–1014
Kawamura M, Heineche JW, Chait A (2000) Increased uptake of alpha-hydroxy aldehyde-modified low density lipoprotein by macrophage scavenger receptors. J Lipid Res 41:1054–1059
Geraldes P, King GL (2010) Activation of protein kinase C isoforms and its impact on diabetic complications. Circ Res 106:1319–1331
Muniandy S, Qvist R, Yan GO et al (2009) The oxidative stress of hyperglycemia and the inflammatory process in endothelial cells. J Mes Invest 56:6–10
Zibaeenezhad MJ, Rezaiezadeh M, Mowla A et al (2003) Antihypertriglyceridemic effect of walnut oil. Angiology 54:411–414
Hardman WE, Ion G (2008) Suppression of implanted MDA-MB 231 human breast cancer growth in nude mice by dietary walnut. Nutr Cancer 60:666–674
Shimoda H, Tanaka J, Kikuchi M et al (2008) Walnut polyphenols prevent liver damage induced by carbon tetrachloride and d-galactosamine: hepatoprotective hydrolyzable tannins in the kernel pellicles of walnut. J Agric Food Chem 56:4444–4449
Cortés B, Núñez I, Cofán M et al (2006) Acute effects of high-fat meals enriched with walnuts or olive oil on postprandial endothelial function. J Am Coll Cardiol 48:1666–16671
Vinson JA, Cai Y (2012) Nuts, especially walnuts, have both antioxidant quantity and efficacy and exhibit significant potential health benefits. Food Funct 3:134–140
Fukuda T, Ito H, Yoshida T (2003) Antioxidative polyphenols from walnuts (Juglans regia L.). Phytochemistry 63:795–801
Berryman CE, Grieger JA, West SG et al (2013) Acute consumption of walnuts and walnut components differentially affect postprandial lipemia, endothelial function, oxidative stress, and cholesterol efflux in humans with mild hypercholesterolemia. J Nutr 143:788–794
Müllner E, Brath H, Pliefer S et al (2013) Vegetables and PUFA-rich plant oil reduce DNA strand breaks in individuals with type 2 diabetes. Mol Nutr Food Res 57:328–338
Anand R, Kaithwas G (2014) Anti-inflammatory potential of alpha-linolenic acid mediated through selective cox inhibition: computational and experimental data. Inflammation [Epub ahead of print]
Mosman T (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 65:55–63
Price P, McMillan JT (1990) Use of the tetrazolium assay in measuring the response of human tumor cells to ionizing radiation. Cancer Res 50:1392–1396
Re R, Pellegrini N, Pannala A et al (1991) Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med 26:1231–1237
Buss IH, Chan TP, Sluis KB et al (1997) Protein carbonyl measurement by a sensitive ELISA method. Free Radic Biol Med 23:361–366
Collins AR, Dobson VL, Dusinska M et al (1997) The comet assay: what can it really tell us? Mutat Res 375:183–193
Garciá A, Serrano A, Abril E et al (1999) Differential effect on U937 cell differentiation by targeting transcriptional factors implicated in tissue- or stage-specific induced integrin expression. Exp Hematol 27:353–364
Jain SK, Kannan K (2001) Chromium chloride inhibits oxidative stress and TNF-a secretion caused by exposure to high glucose in cultured U937 monocytes. Biochem Biophys Res Commun 289:687–691
Guha M, Mackman N (2001) LPS induction of gene expression in human monocytes. Cell Signal 13:85–94
Mander T, Hill S, Hughes A et al (1997) Differential effects on TNF alpha production by pharmacological agents with varying molecular sites of action. Int J Immunopharmacol 19:451–462
Ceriello A, dello Russo P, Amstad P et al (1996) High glucose induces antioxidant enzymes in human endothelial cells in culture: evidence linking hyperglycemia and oxidative stress. Diabetes 45:471–477
Vivancos M, Moreno JJ (2005) Beta-Sitosterol modulates antioxidant enzyme response in RAW 264.7 macrophages. Free Radic Biol Med 39:91–97
Nishikawa T, Sasahara T, Kiritoshi S (2003) Evaluation of urinary 8-hydroxydeoxy-guanosine as a novel biomarker of macrovascular complications in type 2 diabetes. Diabetes Care 26:1507–1512
Dandona P, Thusu K, Cook S et al (1996) Oxidative damage to DNA in diabetes mellitus. Lancet 347:444–445
Shin CS, Moon BS, Park KS et al (2001) Serum 8-hydroxy-guanine levels are increased in diabetic patients. Diabetes Care 24:733–737
Müllner E, Brath H, Tofere D et al (2013) Genome damage in peripheral blood lymphocytes of diabetic and non-diabetic individuals after intervention with vegetables and plant oil. Mutagenesis 28:205–211
Dayanand CD, Vegi PK, Kutty AV (2012) Protein carbonyl content as a stable oxidative stress marker in type II diabetes. Int J Biol Med Res 3:2362–2365
Calabrese V, Cornelius C, Leso V et al (2012) Oxidative stress, glutathione status, sirtuin and cellular stress response in type 2 diabetes. Biochim Biophys Acta 1822:729–736
Du Y, Miller CM, Kern TS (2003) Hyperglycemia increases mitochondrial superoxide in retina and retinal cells. Free Radic Biol Med 35:1491–1499
Ros E (2009) Nuts and novel biomarkers of cardiovascular. Am J Clin Nutr 89:1649–1656
Navarro JF, Mora C (2005) Role of inflammation in diabetic complications. Nephrol Dial Transplant 20:2601–2604
Dandona P, Aljada A, Bandyopadhyay A (2003) The potential therapeutic role of insulin in acute myocardial infarction in patients admitted to intensive care and in those with unspecified hyperglycemia. Diabetes Care 26:516–519
Kim HJ, Kim SH, Yun JM (2012) Fisetin inhibits hyperglycemia-induced pro-inflammatory cytokine production by epigenetic mechanisms. Evid Based Complement Alternat Med 2012:1–10
Burdge GC, Calder PC (2005) Plasma cytokine response during the postprandial period: a potential causal process in vascular disease? Br J Nutr 93:3–9
Yun JM, Jialal I, Devaraj S (2011) Epigenetic regulation of high glucose-induced pro-inflammatory cytokine production in monocytes by curcumin. J Nutr Biochem 22:450–458
Rahman I, Biswas SK, Kirkham PA (2006) Regulation of inflammation and redox signaling by dietary polyphenols. Biochem Pharmacol 72:1439–1452
Papoutsi Z, Kassi E, Chinou I et al (2008) Walnut extract (Juglans regia L.) and its component ellagic acid exhibit anti-inflammatory activity in human aorta endothelial cells and osteoblastic activity in the cell line KS483. Br J Nutr 99:715–722
Singh U, Jialal I (2004) Anti-inflammatory effects of alpha-tocopherol. Ann NY Acas Sci 1031:195–203
Acknowledgments
This study was supported by the Diaplant project N00039 AT-SK, VEGA 1/1133/11 and by the Mind & Health, civil association.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Laubertová, L., Koňariková, K., Gbelcová, H. et al. Effect of walnut oil on hyperglycemia-induced oxidative stress and pro-inflammatory cytokines production. Eur J Nutr 54, 291–299 (2015). https://doi.org/10.1007/s00394-014-0710-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-014-0710-3