Abstract
Purpose
Biological effects of marine oils, fish oil (FO) and krill oil (KO), are mostly attributed to the high content of n-3 polyunsaturated fatty acids (n-3 PUFAs), predominantly eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA). The study was aimed to investigate the influence of FO and KO on lipid homeostasis and inflammation in an animal model of persistent low-grade exposure to human tumor necrosis factor α (hTNF-α) and to evaluate whether these effects depend on the structural forms of EPA and DHA [triacylglycerols (TAG) vs. phospholipids].
Methods
Male C57BL/6 hTNF-α mice were fed for 6 weeks a high-fat control diet (24.50 % total fats, w/w) or high-fat diets containing either FO or KO at similar doses of n-3 PUFAs (EPA: 5.23 vs. 5.39 wt%, DHA: 2.82 vs. 2.36 wt% of total fatty acids).
Results
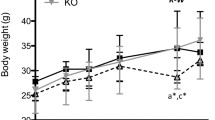
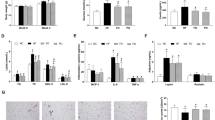
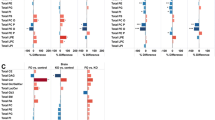
We found that KO, containing bioactive n-3 PUFAs in the form of phospholipids, was capable of modulating lipid metabolism by lowering plasma levels of TAG and cholesterol and stimulating the mitochondrial and peroxisomal fatty acid β-oxidation, as well as improving the overall carnitine turnover. Though the administration of FO was not as effective as KO in the lowering of plasma TAG, FO significantly improved the levels of all cholesterol classes in plasma. Except from the increase in the levels of IL-17 in FO-fed mice and a trend to decrease in MCP-1 levels in KO-fed animals, the levels of pro-inflammatory cytokines were not substantially different between treatment groups.
Conclusion
Our findings demonstrate that FO and KO are comparable dietary sources of n-3 PUFAs. However, when quantitatively similar doses of n-3 PUFAs are administered, KO seems to have a greater potential to promote lipid catabolism. The effect of dietary oils on the levels of inflammatory markers in hTNF-α transgenic mice fed a high-fat diet needs further investigations.




Similar content being viewed by others
Abbreviations
- ACOX1:
-
Acyl-CoA oxidase 1
- CPT:
-
Carnitine palmitoyltransferase
- DHA:
-
Docosahexaenoic acid
- EPA:
-
Eicosapentaenoic acid
- FFA:
-
Free fatty acids
- GPAT:
-
Glycerol phosphate acyltransferase
- HDL:
-
High-density lipoprotein
- HPLC:
-
High-performance liquid chromatography
- LDL:
-
Low-density lipoprotein
- MUFA:
-
Monounsaturated fatty acid
- PPAR:
-
Peroxisome proliferator-activated receptor
- PUFA:
-
Polyunsaturated fatty acid
- SFA:
-
Saturated fatty acid
- TAG:
-
Triacylglycerol
- TNF-α:
-
Tumor necrosis factor α
- VLDL:
-
Very low-density lipoprotein
References
Whelton SP, He J, Whelton PK, Muntner P (2004) Meta-analysis of observational studies on fish intake and coronary heart disease. Am J Cardiol 93(9):1119–1123
He K, Song Y, Daviglus ML, Liu K, Van Horn L, Dyer AR, Goldbourt U, Greenland P (2004) Fish consumption and incidence of stroke: a meta-analysis of cohort studies. Stroke 35(7):1538–1542
Deutsch L (2007) Evaluation of the effect of Neptune Krill Oil on chronic inflammation and arthritic symptoms. J Am Coll Nutr 26(1):39–48
Bunea R, El Farrah K, Deutsch L (2004) Evaluation of the effects of Neptune Krill Oil on the clinical course of hyperlipidemia. Altern Med Rev 9(4):420–428
Deckelbaum RJ, Worgall TS, Seo T (2006) n-3 fatty acids and gene expression. Am J Clin Nutr 83(6 Suppl):1520S–1525S
Howe PR, Clifton PM, James MJ (1999) Equal antithrombotic and triglyceride-lowering effectiveness of eicosapentaenoic acid-rich and docosahexaenoic acid-rich fish oil supplements. Lipids 34(Suppl):S307–S308
Mori TA, Woodman RJ (2006) The independent effects of eicosapentaenoic acid and docosahexaenoic acid on cardiovascular risk factors in humans. Curr Opin Clin Nutr Metab Care 9(2):95–104
Tou JC, Jaczynski J, Chen YC (2007) Krill for human consumption: nutritional value and potential health benefits. Nutr Rev 65(2):63–77
Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW Jr (2003) Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest 112(12):1796–1808
Berg AH, Scherer PE (2005) Adipose tissue, inflammation, and cardiovascular disease. Circ Res 96(9):939–949
Hotamisligil GS, Arner P, Caro JF, Atkinson RL, Spiegelman BM (1995) Increased adipose tissue expression of tumor necrosis factor-alpha in human obesity and insulin resistance. J Clin Invest 95(5):2409–2415
Hotamisligil GS, Shargill NS, Spiegelman BM (1993) Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science 259(5091):87–91
Shoelson SE, Lee J, Goldfine AB (2006) Inflammation and insulin resistance. J Clin Invest 116(7):1793–1801
Cai D, Yuan M, Frantz DF, Melendez PA, Hansen L, Lee J, Shoelson SE (2005) Local and systemic insulin resistance resulting from hepatic activation of IKK-beta and NF-kappaB. Nat Med 11(2):183–190
De Souza CT, Araujo EP, Bordin S, Ashimine R, Zollner RL, Boschero AC, Saad MJ, Velloso LA (2005) Consumption of a fat-rich diet activates a proinflammatory response and induces insulin resistance in the hypothalamus. Endocrinology 146(10):4192–4199
Baffy G (2009) Kupffer cells in non-alcoholic fatty liver disease: the emerging view. J Hepatol 51(1):212–223
Beier K, Volkl A, Fahimi HD (1992) Suppression of peroxisomal lipid beta-oxidation enzymes of TNF-alpha. FEBS Lett 310(3):273–276
Feingold KR, Grunfeld C (1987) Tumor necrosis factor-alpha stimulates hepatic lipogenesis in the rat in vivo. J Clin Invest 80(1):184–190
Glosli H, Gudbrandsen OA, Mullen AJ, Halvorsen B, Rost TH, Wergedahl H, Prydz H, Aukrust P, Berge RK (2005) Down-regulated expression of PPARalpha target genes, reduced fatty acid oxidation and altered fatty acid composition in the liver of mice transgenic for hTNFalpha. Biochim Biophys Acta 1734(3):235–246
Berge RK, Flatmark T, Osmundsen H (1984) Enhancement of long-chain acyl-CoA hydrolase activity in peroxisomes and mitochondria of rat liver by peroxisomal proliferators. Eur J Biochem (FEBS) 141(3):637–644
Madsen L, Garras A, Asins G, Serra D, Hegardt FG, Berge RK (1999) Mitochondrial 3-hydroxy-3-methylglutaryl coenzyme A synthase and carnitine palmitoyltransferase II as potential control sites for ketogenesis during mitochondrion and peroxisome proliferation. Biochem Pharmacol 57(9):1011–1019
Madsen L, Froyland L, Dyroy E, Helland K, Berge RK (1998) Docosahexaenoic and eicosapentaenoic acids are differently metabolized in rat liver during mitochondria and peroxisome proliferation. J Lipid Res 39(3):583–593
Skorve J, al-Shurbaji A, Asiedu D, Bjorkhem I, Berglund L, Berge RK (1993) On the mechanism of the hypolipidemic effect of sulfur-substituted hexadecanedioic acid (3-thiadicarboxylic acid) in normolipidemic rats. J Lipid Res 34(7):1177–1185
Bates EJ, Saggerson D (1977) A selective decrease in mitochondrial glycerol phosphate acyltransferase activity in livers from streptozotocin-diabetic rats. FEBS Lett 84(2):229–232
Bligh EG, Dyer WJ (1959) A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37(8):911–917
Grimstad T, Bjorndal B, Cacabelos D, Aasprong OG, Janssen EA, Omdal R, Svardal A, Hausken T, Bohov P, Portero-Otin M, Pamplona R, Berge RK (2012) Dietary supplementation of krill oil attenuates inflammation and oxidative stress in experimental ulcerative colitis in rats. Scand J Gastroenterol 47(1):49–58
Vernez L, Wenk M, Krahenbuhl S (2004) Determination of carnitine and acylcarnitines in plasma by high-performance liquid chromatography/electrospray ionization ion trap tandem mass spectrometry. Rapid Commun Mass Spectrom 18(11):1233–1238
Ulven SM, Kirkhus B, Lamglait A, Basu S, Elind E, Haider T, Berge K, Vik H, Pedersen JI (2011) Metabolic effects of krill oil are essentially similar to those of fish oil but at lower dose of EPA and DHA, in healthy volunteers. Lipids 46(1):37–46
Burri L, Berge K, Wibrand K, Berge RK, Barger JL (2011) Differential effects of krill oil and fish oil on the hepatic transcriptome in mice. Front Genet 2:45
Batetta B, Griinari M, Carta G, Murru E, Ligresti A, Cordeddu L, Giordano E, Sanna F, Bisogno T, Uda S, Collu M, Bruheim I, Di Marzo V, Banni S (2009) Endocannabinoids may mediate the ability of (n-3) fatty acids to reduce ectopic fat and inflammatory mediators in obese Zucker rats. J Nutr 139(8):1495–1501
Meydani SN, Endres S, Woods MM, Goldin BR, Soo C, Morrill-Labrode A, Dinarello CA, Gorbach SL (1991) Oral (n-3) fatty acid supplementation suppresses cytokine production and lymphocyte proliferation: comparison between young and older women. J Nutr 121(4):547–555
Kelley DS, Taylor PC, Nelson GJ, Schmidt PC, Ferretti A, Erickson KL, Yu R, Chandra RK, Mackey BE (1999) Docosahexaenoic acid ingestion inhibits natural killer cell activity and production of inflammatory mediators in young healthy men. Lipids 34(4):317–324
Blok WL, Deslypere JP, Demacker PN, van der Ven-Jongekrijg J, Hectors MP, van der Meer JW, Katan MB (1997) Pro- and anti-inflammatory cytokines in healthy volunteers fed various doses of fish oil for 1 year. Eur J Clin Invest 27(12):1003–1008
Schmidt EB, Varming K, Moller JM, Bulow Pedersen I, Madsen P, Dyerberg J (1996) No effect of a very low dose of n-3 fatty acids on monocyte function in healthy humans. Scand J Clin Lab Invest 56(1):87–92
Dewell A, Marvasti FF, Harris WS, Tsao P, Gardner CD (2011) Low- and high-dose plant and marine (n-3) fatty acids do not affect plasma inflammatory markers in adults with metabolic syndrome. J Nutr 141(12):2166–2171
Koenders MI, Lubberts E, van de Loo FA, Oppers-Walgreen B, van den Bersselaar L, Helsen MM, Kolls JK, Di Padova FE, Joosten LA, van den Berg WB (2006) Interleukin-17 acts independently of TNF-alpha under arthritic conditions. J Immunol 176(10):6262–6269
Popa C, Netea MG, van Riel PL, van der Meer JW, Stalenhoef AF (2007) The role of TNF-alpha in chronic inflammatory conditions, intermediary metabolism, and cardiovascular risk. J Lipid Res 48(4):751–762
Ukropec J, Reseland JE, Gasperikova D, Demcakova E, Madsen L, Berge RK, Rustan AC, Klimes I, Drevon CA, Sebokova E (2003) The hypotriglyceridemic effect of dietary n-3 FA is associated with increased beta-oxidation and reduced leptin expression. Lipids 38(10):1023–1029
Hoppel C (2003) The role of carnitine in normal and altered fatty acid metabolism. Am J Kidney Dis 41(4 Suppl 4):S4–S12
Ferramosca A, Conte L, Zara V (2012) A krill oil supplemented diet reduces the activities of the mitochondrial tricarboxylate carrier and of the cytosolic lipogenic enzymes in rats. J Anim Physiol Anim Nutr (Berl) 96(2):295–306
Harris WS (1997) n-3 fatty acids and serum lipoproteins: human studies. Am J Clin Nutr 65(5 Suppl):1645S–1654S
Balk EM, Lichtenstein AH, Chung M, Kupelnick B, Chew P, Lau J (2006) Effects of omega-3 fatty acids on serum markers of cardiovascular disease risk: a systematic review. Atherosclerosis 189(1):19–30
Vigerust NF, Cacabelos D, Burri L, Berge K, Wergedahl H, Christensen B, Portero-Otin M, Viste A, Pamplona R, Berge RK, Bjorndal B (2012) Fish oil and 3-thia fatty acid have additive effects on lipid metabolism but antagonistic effects on oxidative damage when fed to rats for 50 weeks. J Nutr Biochem. doi:10.1016/j.jnutbio.2011.08.006
Tandy S, Chung RW, Wat E, Kamili A, Berge K, Griinari M, Cohn JS (2009) Dietary krill oil supplementation reduces hepatic steatosis, glycemia, and hypercholesterolemia in high-fat-fed mice. J Agric Food Chem 57(19):9339–9345
Acknowledgments
We thank Liv Kristine Øysæd, Kari Williams, Randi Sandvik, Torunn Eide and Svein Krüger for excellent technical assistance. This work was supported by grants from Nordforsk, grant 070010, MitoHealth and the Research Council of Norway, grant 190287/110.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vigerust, N.F., Bjørndal, B., Bohov, P. et al. Krill oil versus fish oil in modulation of inflammation and lipid metabolism in mice transgenic for TNF-α. Eur J Nutr 52, 1315–1325 (2013). https://doi.org/10.1007/s00394-012-0441-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-012-0441-2